A Bidomain Model for Lens Microcirculation
Abstract
There exists a large body of research on the lens of mammalian eye over the past several decades. The objective of the current work is to provide a link between the most recent computational models and some of the pioneering work in the 1970s and 80s. We introduce a general non-electro-neutral model to study the microcirculation in lens of eyes. It describes the steady state relationships among ion fluxes, water flow and electric field inside cells, and in the narrow extracellular spaces between cells in the lens. Using asymptotic analysis, we derive a simplified model based on physiological data and compare our results with those in the literature. We show that our simplified model can be reduced further to the first generation models while our full model is consistent with the most recent computational models. In addition, our simplified model captures the main features of the full model. Our results serve as a useful link intermediate between the computational models and the first generation analytical models. Simplified models of this sort may be particularly helpful as the roles of similar osmotic pumps of microcirculation are examined in other tissues with narrow extracellular spaces, like cardiac and skeletal muscle, liver, kidney, epithelia in general, and the narrow extracellular spaces of the central nervous system, the “brain”. Simplified models may reveal the general functional plan of these systems before full computational models become feasible and specific.
1 Introduction
Biological systems require continual inputs of mass and energy to stay alive. They are open systems that require flow of matter, and specific chemicals, across their boundaries. Unicellular organisms can provide that flow by diffusion to and across cell membranes. Diffusion is not adequate over distances larger than a few cell diameters, i.e., larger than say meters, to pick a number. For that reason, multicellular organisms cannot provide those flows to their cells by diffusion itself. Multicellular organisms depend on convection to bring materials close enough to cells so diffusion to and across cell membranes can provide what the cell needs to live.
The circulatory system of blood vessels-arteries, veins, and capillaries-provides the convection in almost all tissues. But there is one clear exception, the lens of the (mammalian) eye. The lens does not have blood vessels, presumably because even capillaries would so seriously interfere with transparency. The lens is large, much larger than the length scale on which diffusion itself is efficient. The lens must provide nutrients through another kind of convection, a microcirculation of water that moves nutrients into the lens and rinses wastes out of it. The microcirculation is in fact driven by the lens itself, without an external ‘pump’. The lens is itself an osmotic pump.
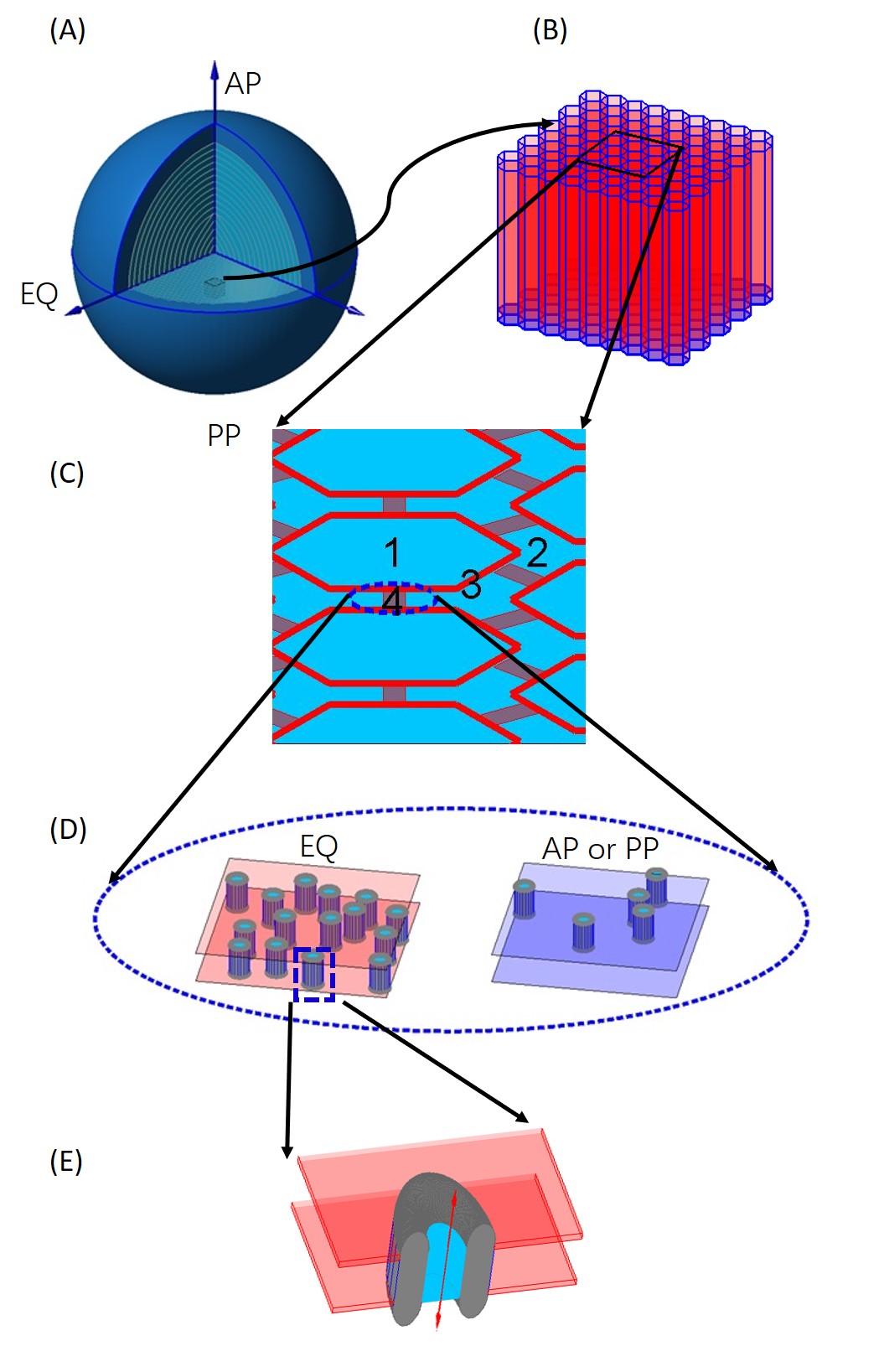
The lens is an asymmetrical electrical syncytium in which all cells are electrically coupled one to another, with a narrow extracellular space between the cells (see Fig.1). The extracellular space is filled with ionic solution in ‘free diffusion’ with the plasma outside cells. It may also contain specialized more or less immobile proteins and specialized polysaccharides, as well as containing obstructions formed by the connexin proteins themselves. The intracellular space behaves very much as a large single cell would, with the bio-ions of classical electro-physiology free to move without much resistance from cell to cell, and many solutes of significant size (say with diameter less than 1.5 nm) able to move as well. The intracellular media contains proteins particularly the crystallins responsible for the high refractive index of the lens. So the lens is an example of a bidomain tissue that has been studied in some detail, first in skeletal muscle, then in cardiac muscle, and syncytia in general. Electrical models of bidomain tissues have been developed and a general approach combining morphology, theory, and experiments has been applied in reference [1], showing how the lens could be studied in this tradition.
A general approach to bidomain tissues was implemented [2] involving detailed measurements of morphology (best done with statistical sampling by stereo-logical methods [3]), impedance spectroscopy [4, 5, 6, 7, 8, 9, 10] using intracellular probes (micro-electrodes) that force current to flow across membranes to the extracellular baths [11, 1, 12, 13, 14, 15], electric field theory to develop models appropriate to the structure [16, 17, 18, 19, 20] analyzing the spectroscopic data with the field theory [21, 22] and checking that parameters change appropriately (i.e., estimates of membrane capacitance are constant) as extracellular solutions are changed in composition and concentration [20, 23]. This work was extended to deal with transport by Mathias and co-workers [24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40] and computational models of the water flow in the lens were later developed in some detail [25, 41, 42] and exploited with great success, reviewed in [43, 44], also see [45, 46, 47, 48, 49].
The original work on electrical models is cited here because it provides coherent support, involving a range of techniques and approaches, to the general view of syncytial tissues, used here and in later work. It also shows the range of approaches needed to establish a (then) new view of a tissue.
Mathias [50, 51] realized that an asymmetrical electrical syncytium would produce convection, in particular in the lens [31]: he and co-workers systematically investigated the flow of water, solutes, and current in the lens, which is (in our opinion) a model of interdisciplinary research, combining theory, simulation, and measurements of many types [24, 25, 26, 27, 28, 29, 30, 32, 33, 34, 35, 36, 37, 38, 39, 40]. Computational models of the water flow in the lens were later developed in great detail [25, 41, 42] and compared to the more analytical models. These models have been extensively tested and we are fortunate that comprehensive reviews have been written of great value to newcomers to the field, particularly [43, 44] as well as [45, 46, 47, 48, 49, 43, 41, 44].
Since the pioneering work on the models of lens microcirculation system proposed by Mathias et al. [51, 21], numerous investigations have been carried out [52, 53, 23, 20, 19]. The microcirculation model has firstly relied on a combination of electrical resistance and current measurements and theoretical modeling [18, 54, 19]. More recently, in order to provide a better understanding the electric current flow and potential field, the detail structure of lens has been included [55, 41, 44, 43], describing the asymmetric biological properties of the lens and measurements of pressure have been made [47, 28]. Different types of fluid flow [56, 57] and transport properties of the ions have been introduced. Meanwhile, the lens model [55] has been extended to simulate age-related changes in lens physiology [58] and a variety of physiological processes [26, 59, 60, 61]. Reviews of current studies on micro-circulation in lens are most helpful [30, 26, 33]. Despite this large body of experimental and theoretical work, it is not completely clear how they are related to each other. In particular, it is not clear how the latest computational models are related to the pioneering work, and how theoretical analysis is related to experimental findings. In this paper, we will provide such a link.
Based on the microscale model for semipermeable membrane [62] and bidomain method [51], we construct a mathematical model to ensure that all interactions are included and treated consistently. Using asymptotic analysis, we derive a reduced model, which can be used to obtain most physiologically significant quantities except for the intracellular pressure. This simplified model can be further reduced to the model proposed by Mathias [51] with additional assumptions that Nernst potentials (that describe gradients of chemical potential of each ionic species) and conductance are are constant in space. However, we will show that neither the Nernst potentials nor the conductance are constant. On the contrary, they vary significantly from the interior to the surface of the lens Therefore, both of these quantities need to be coupled as part of the solution.
Our model also shows explicitly that the intracellular pressure is decoupled from the rest of the variables. Evolution has chosen parameters so the intracellular pressure does not affect the other parameters of the lens in a significant way. They are robust to variations of intracellular pressure. The evolutionary advantage of this adaptation is not clear to us, but may be more obvious to other workers with a greater knowledge of clinical realities that show how the lens becomes diseased [63, 46, 48, 49]. Our simplified model suggests that all the quantities can be computed without knowing the intracellular pressure. On the other hand, we need to solve the full model to find the value of the intracellular pressure. Our model is also calibrated by experimental data and predicts the effects of gap junctions [28, 47] described by a ‘membrane’ permeability .
Our new results extend but do not fundamentally change previous work on the lens. We strengthen the view that the lens provides an osmotic pump to maintain the microcirculation necessary to sustain a living lens, for the life of the animal. We imagine that similar osmotic pumps create microcirculation in other cells and tissues of the body.
This paper is organized as following. The full model for micro-circulation of water and ions are proposed based on conservation laws in Section 2. In Section 3, we obtain the leading order model by identifying small parameters in the full model. Based on the boundary conditions and partial differential equation (PDE) analysis, a simplified version of the leading order model is proposed and compared with the existing models. The model calibration and simulation results are shown in Section 4. The conclusions and future work are given in Section 5.
2 Mathematical model
In this section, we present a 1-D spherical symmetric non-electro-neutral model for microcirculation of the lens with radius by using the bidomain method [17]. The model deals with two types of flow: the circulation of water (hydrodynamics) and the circulation of ions (electrodynamics), generalizing previous bidomain models that deal only with electrodynamics. The model is mainly derived from laws of conservation of ions and water in the presence of membrane flow between intra and extracellular domains. We note that a similar approach may be useful in other tissues with narrow extracellular space, like the heart, cardiac muscle, and the central nervous system, including the cerebral cortex, ‘the brain’.
2.1 Water circulation
We assume
-
1.
the loss of intracellular water is only through membranes flowing into the extracellular space, vice versa [17];
-
2.
the trans-membrane water flux is proportional to the intra/extra-cellular hydrostatic pressure and osmotic pressure differences, i.e. Starling’s law, classically applied to capillaries, here applied to membranes [64]. In a system like non-ideal ionic solutions in which ‘everything interacts with everything else’ [65, 66], this statement needs derivation as well as assertion. A complete, and rigorous derivation can be found in [62];
-
3.
in the rest of this paper, subscript denotes the intra-/extracellular space and superscript Na+, K+, Cl- denotes the th specie ion.
Then we obtain the following system for intra and extracellular velocities in domain
| (1a) | |||
| (1b) | |||
where and are the velocity and pressure in the intracellular and extracellular space, respectively. And is the osmotic pressure with definition
where is the concentration of th specie ion in space. is the density of the permanent negative charged protein. In this paper, we assume the permanent negative charged protein is uniformly distributed within intracellular space with valence of . Here is the ratio of intracellular area and extracellular area , is the membrane area per volume unit, is the intracellular membrane reflectance, is intracellular membrane hydraulic permeability, is Boltzmann constant and is temperature.
As we mentioned before, the intracellular space is a connected space, where water can flow from cell to cell through connexin proteins joining membranes of neighboring cells,, and the extracellular space is narrow with a high tortuosity. The intracellular velocity depends on the gradients of hydrostatic pressure and osmotic pressure [62, 41, 51], and the extracellular velocity is determined by the gradients of hydrostatic pressure and electric potential [41, 67],
| (2a) | |||
| (2b) | |||
where is the electric potential in the space, is the tortuosity of extracellular region and is the viscosity of water, is introduced to describe the effect of electro osmotic flow, is the permeability of intracellular region () and extracellular region (), respectively.
Thanks to Eq. 2, Eq.1 can be treated as equation of hydraulic pressure. Due to the axis symmetry condition, homogeneous Neumann boundary conditions are used for pressure at . At the surface of lens , we set the extracellular hydrostatic pressure to be zero and the intracellular velocity is consistent with Eq.2
| (3) |
where is surface membrane reflectance and is surface membrane hydraulic permeability.
2.2 Ion circulation
With similar assumptions, the conservation of ion concentration yields the following ion flux system
| (4a) | |||
| (4b) | |||
The ion flux in the intracellular region and ion flux in the extracellular region are defined as
| (5a) | |||
| (5b) | |||
where is the diffusion coefficient of the th specie ion in the space. The Hodgkin-Huxley conductance formulation [68, 69] is used to describe the trans-membrane flux of ions across intracellular membrane and surface membrane
| (6a) | |||
| (6b) | |||
where is the Nernst potential (an expression of the difference of chemical potential ) of th specie ion.
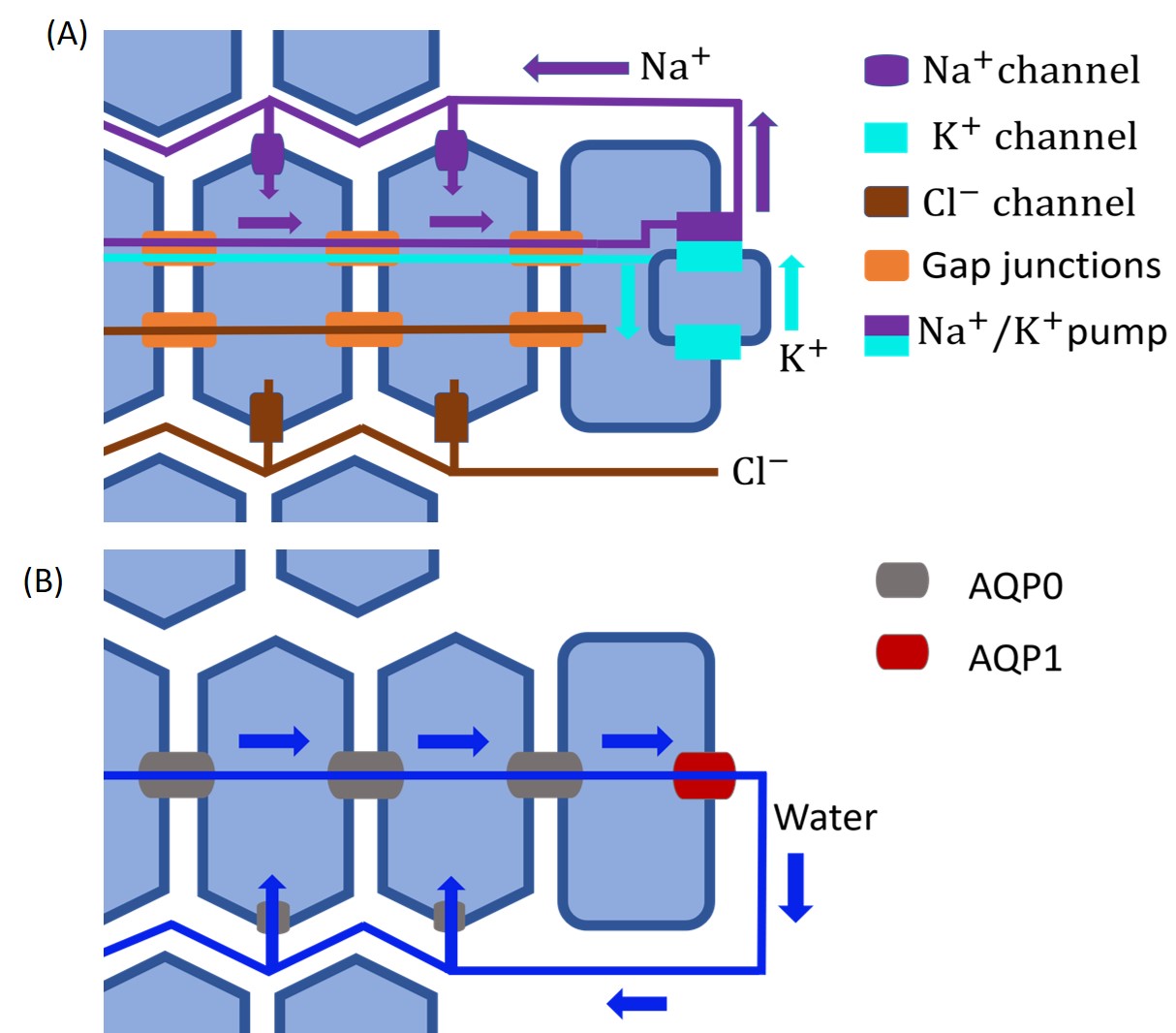
In Eq. 6, the intracellular ion conductance and surface ion conductance depends on the ion channel distribution on the membrane (see Fig. 2). Based on previous work [51, 17, 41], we assume that (1) only Na+ and Cl- can leak between intracellular and extracellular through ion channels inside the lens and (2) that there is no trans-membrane flux for K+ between the extracellular and intracellular region, i.e. .
Similarly, homogeneous Neumann boundary conditions are used at . At , the extracellular concentrations are fixed and Robin boundary conditions are used for intracellular concentrations due to the trans-membrane flux and pump,
| (7) |
where is active ion pump on the surface membrane. Here we only consider the sodium-potassium pump on the surface. The strength of the pump depends on the ion’s concentration as in [29, 41],
| (8) |
where
| (9) | ||||
Due to the capacitance of the cell membrane, assumptions of exact charge neutrality can easily lead to paradoxes because they oversimplify Maxwell’s equations by leaving out altogether the essential role of charge. We use the analysis of [70] and thus introduce a linear correction term replacing the charge neutrality condition [51, 41], without introducing significant error (See also [71]),
| (10a) | |||
| (10b) | |||
where is the porosity of intracellular region and is capacitance per unit area.
Multiplying each ion concentration equation in Eq. 4 with respectively, summing up and using Eq.10, the sodium equations are replaced by the following equations
| (11a) | |||
| (11b) | |||
with boundary conditions
where and
In Eq. 11, we define the intracellular conductance and extracellular conductance as
It is obvious that system 11 might be derived using either Eq. 4 and Eq. 10. Therefore, we should drop either Eq. 4 or Eq. 10 when Eq. 11 is used.
2.3 Non-dimensionalization
Since lens circulation is driven by the sodium-potassium pump, it is natural to choose the characteristic velocity by the pump strength
| (12) |
where is characteristic osmotic pressure. Using Eq.1b, we obtain the scale of as
| (13) |
where . With the characteristic values for , , , chosen as , , , we obtain the dimensionless system for lens problem as follows ( Detailed derivation is given in the appendix C.)
| (14a) | |||
| (14b) | |||
| (14c) | |||
| (14d) | |||
| (14e) | |||
| (14f) | |||
| (14g) | |||
| (14h) | |||
| (14i) | |||
| (14j) | |||
| (14k) | |||
| (14l) | |||
with homogeneous Neumann boundary conditions at and following boundary conditions at
where
| (15a) | |||
| (15b) | |||
| (15c) | |||
| (15d) | |||
| (15e) | |||
| (15f) | |||
3 Simplified model
The full model given by system 14 with boundary condition LABEL:fullmodelndbd is a coupled nonlinear system. In this section, we present a simplified version of the full model which captures the main features of the lens circulation. We first obtain the leading order model by identify the small parameters. And then by using boundary conditions and theoretical analysis, the leading order model with is further simplified as only one PDE with serial algebra equations.
According to those dimensionless parameters presented in the appendix B, we identify the scale of the parameters as follows
| (16) | ||||
If we denote and , , it yields
| (17) |
3.1 A priori estimation
In this section, we provide the priori estimation of the as follows. By using the homogeneous Neumann boundary condition at and Eq. 14l yields
| (18) | ||||
From Eq. 18, since and order of , , in Eqs. LABEL:SmP-17, we obtain that
| (19) |
Meanwhile, from Eq. 14b we can have
| (20) |
and in the Eq.14e, we know
| (21) |
With Eqs. 20-21 and is constants, we obtain
| (22) |
Furthermore, using Eq. 14d and boundary conditions for in Eq. LABEL:fullmodelndbd yieldds
| (23) |
From the experimental setting of lens [51, 55, 41], we assume that
| (24) |
Therefore,
| (25) |
In all, we claim that
| (26) | ||||
By dropping the terms involving these small parameters, the leading order of water circulation system 14a-14d is as follows,
| (27a) | |||
| (27b) | |||
| (27c) | |||
| (27d) | |||
where the superscript ‘0’ denotes the leading order approximation. From Eq. 27, we deduce are constants, and the intracellular and extracellular flow are counterflow. And the total charge in the leading order systems are neutral
| (28a) | |||
| (28b) | |||
Combining constant osmotic pressure and charge neutrality yields
| (29a) | |||
| (29b) | |||
which means and are constants and
| (30) |
And the leading order of potassium and chloride concentrations satisfy
| (31a) | |||
| (31b) | |||
| (31c) | |||
| (31d) | |||
where with and
For the electric potential, using the homogeneous Neumann boundary condition at and Eqs. 29a -30, 14l yields
| (32) | |||
At the same time, based on the intracellular equation of potassium Eq. 14j the homogeneous Neumann boundary condition at and Eqs. 29a -30, we have
| (33) |
Substituting Eq. 32 into Eq. 33 yields
| (34) | |||||
where we used that fact that , and Since , and , in Eq. 34, we claim
| (35) |
Combining Eqs. 32 and 35 yields the leading order approximation of intracellular potential
| (36) |
where ,
Similarly, the leading order approximation of extracellular potential is
|
|
(37) |
where .
To summarize, the leading order approximation of system 14-LABEL:fullmodelndbd is given by, in domain
3.2 Relation between and
3.3 Relation between and
By the homogeneous Neumann boundary condition on and Eq. 38j, we have
| (41) |
By Eq. 29b, we can divide Eq. 41 by on both sides, we get
|
|
(42) |
3.4 Expression of
3.5 Extracellular electric potential system
By Eqs. LABEL:r1 and 45, we have as
| (49) |
The value is determined by the boundary condition of in Eq. 39, where
| (50) |
where we use
To summarize, we obtained the simplified model of system 38-39 as follows
| (51a) | |||
| (51b) | |||
| (51c) | |||
| (51d) | |||
| (51e) | |||
| (51f) | |||
| (51g) | |||
| (51h) | |||
| (51i) | |||
| (51j) | |||
| (51k) | |||
| (51l) | |||
with boundary conditions
| (52) |
Remark 3.1.
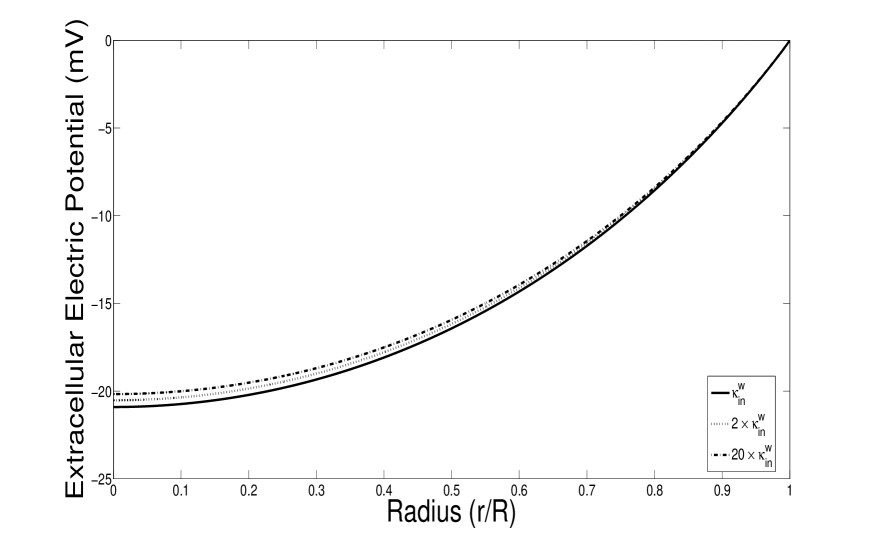
Under the same assumptions in [51], for example, uniform diffusion constants for all ions, constant Nernst potential, our simplified model system 51 recovers the model proposed by Mathias. The main contribution here is that we remove the assumptions that Nernst potentials and effective conductance should be constants. By using the relationships between ions concentrations and external potential, we obtain the space dependent Nernst potential which yields a much better approximation to the full model (see Fig. 4).
4 Results and discussion
In this section, we present numerical simulations using both the full and simplified models. Finite Volume Method [70] is used in order to preserve mass conservation of ions. The convex iteration [72] is employed to solve the nonlinear coupled system. The numerical algorithm is implemented in Matlab.
4.1 Model calibration: membrane conductance effects intracellular hydrostatic pressure
In this section, we first calibrate the full model by the comparing with the experimental data to study effect of connexin to intracellular hydrostatic pressure.
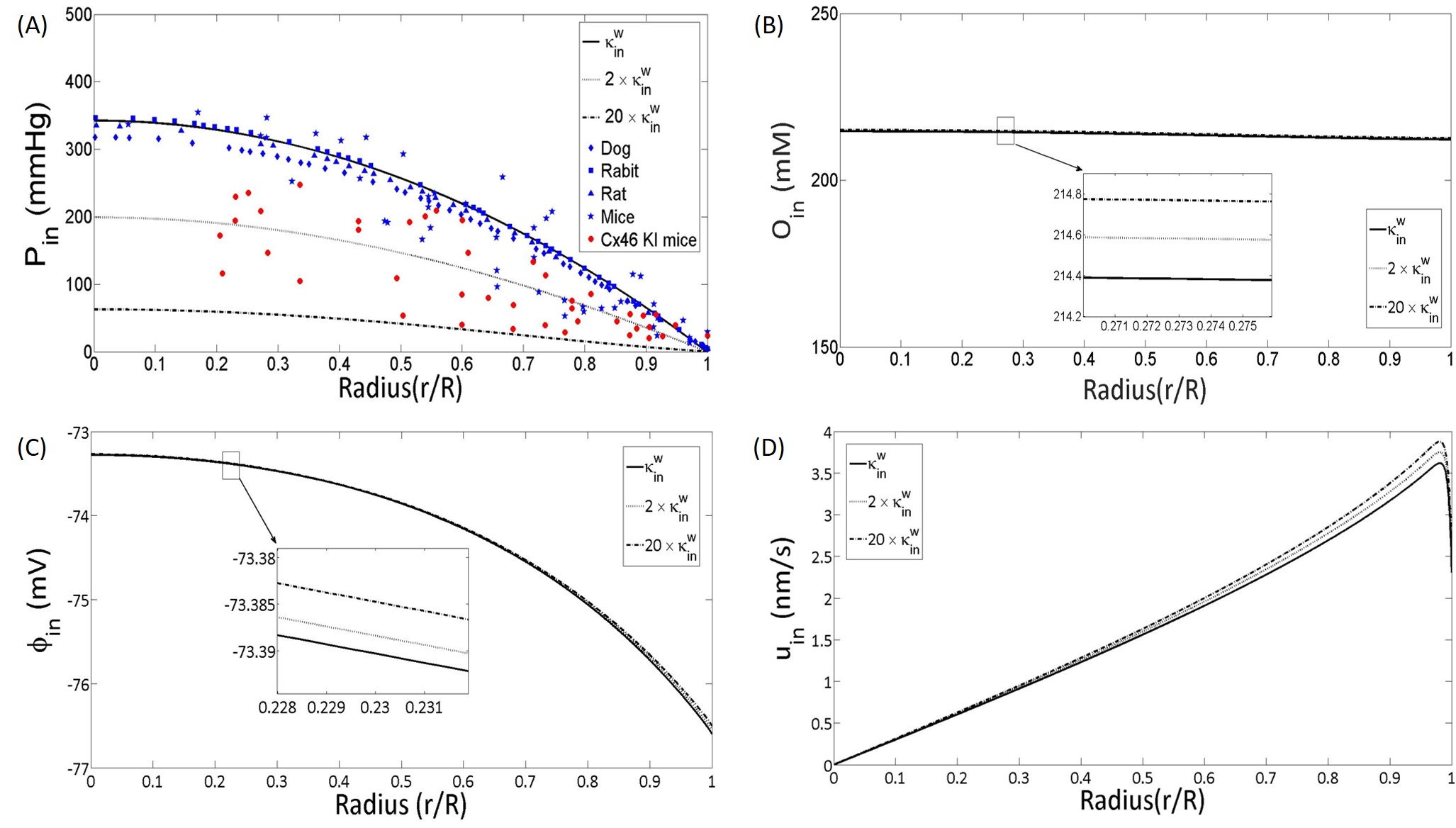
Intracellular hydrostatic pressure is an important physiological quantity [63]. In the paper [28, 47], the authors showed the connexin (gap junction) conductance play an important role in the microcirculation of lens. It is said that if the intracellular conductance in lenses is approximately doubled, the hydrostatic pressure gradient in the lenses should become approximately half of the original one. In this section, we calibrate our model. We choose a value of the intracellular conductance () that correctly calculates the experimental results in the [28, 47].
In Figure 3 (A), the value (black line) yields a good approximation to experimental data (black makers). When the conductivity of the connexins is doubled, to parameter value to be (in the lens of mice Cx46 KI lens) as in the experiments [28, 47], where doubled the conductivity of the connexins by using Cx46 KI mice lens, our model (black dot) can also match the experimental data (red markers): the intracellular hydrostatic pressure drops to half. This result shows that our full model can correctly predict the effect of permeability of membrane on hydrostatic pressure.
Interestingly, panels (B)-(D) it shows that other intracellular quantities and extracellular ones (appendix) are insensitive to increases in the permeability by a factor of twenty, even to . The reason for this can be explained by using our simplified the system 51. If the variation of intracellular conductance still keep the to be a small quantity in the dimensionless system 14, our simplified model will be still valid. In the simplified model, All the quantities except intracellular hydrostatic pressure are related to the extracellular electric potential. However, the extracellular electric potential will not be effected by the change of the intracellular conductance, since Eq. 51a not involves intracellular conductance.
4.2 Full model vs simplified model
In this section, we compare the full model 14-LABEL:fullmodelndbd with the simplified model 51 and Mathias model in [51]. The numerical results of full model (Black lines) in Figure 4 (A-C) suggest that the variations intracellular electric potential, extracellular conductance and Nernst potential of Cl- are rather small. The assumption of constant values for those variables ( potential, extracellular conductance, and Nernst, i.e. chemical potential of Cl- ) in the Mathias’s model (shown as red dash-dot lines) is reasonable. However, the Nernst potentials of sodium and potassium (Figure 4 (D-E)) have large variations, because of the effect of Sodium-potassium pump. Our simplified model (black dash lines) describes these variations with small errors. The comparisons for extracellular pressure, velocity, and potential (Figure 4 (F-H)) confirm that our simplified model yields good approximations to the full model.
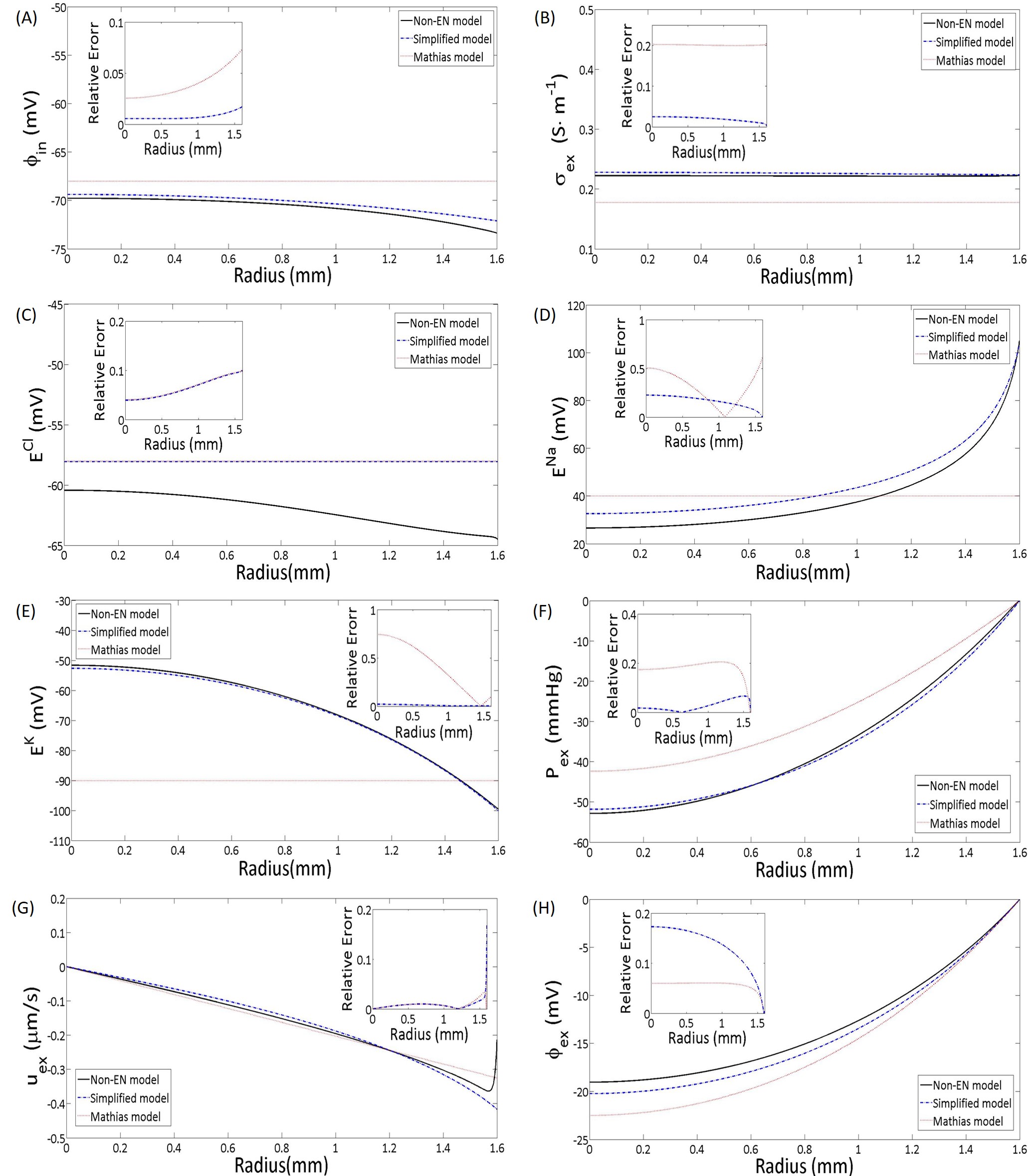
5 Conclusion
In this paper, we propose a bidomain model to study the microcirculation of lens. We include a capacitor in the representation of the membrane and so our model is consistent with classical electrodynamics. Consistency produces a linear correction term in the classical charge neutrality equation. This full model is calibrated by comparing with the experiment studying effect of connexin on hydrostatic pressure. It shows that only by changing intracellular membrane conductance (strength of connexion), our model could match the two experimental results with different connexin very well. Our model is capable of making prediction to the circulation of lens. Furthermore, the numerical simulations show that the velocity, potential, osmotic pressure in the intra and extra cellular are not sensitive to increasing conductance.
Based on the asymptotic analysis, we proposed a simplified model, which allows us to obtain a deep understanding of the physical process without making unrealistic assumptions. Our results showed that the simplified model is a good approximation of the full model where Nernst potentials and conductivity vary significantly inside the lens.
Our model allows calculation of variables that determine the role and life of the lens as an organ. Particularly important are the factors that determine the transparency of the lens, since that is the main function of the organ. The dependence of the size of the extracellular space, and thus the pressure in the extracellular and intracellular spaces and the difference between those two, is likely to be an important determinant of transparency. One imagines that swelling of the extracellular space will scatter light, particularly because the swelling is likely to be irregular (in a way our model does not yet capture). Changes in the Osmolarity (i.e., activity of water estimated by the total concentration of solutes) is likely to be important as well.
This hydrodynamic bidomain model can point the way to dealing with other cells, tissues, and organs in which current flow, water flow, and cell volume changes are important. These include the kidney, the central nervous system (where the narrow extracellular space poses many of the biological problems facing the lens), the t-tubular system of skeletal and much cardiac muscle and so on. We show that a mathematically well defined model can deal with the reality of biological structure and its complex distribution of channels, etc.
Conservation laws applied to simplified structures are enough to provide quite useful results, as they were in three dimensional electrical problems of cells of various geometries [16] and syncytia [3]-[23]. The exact results are analyzed with perturbation methods, described in general in [73] and these methods allow dramatic simplifications without introducing large or even significant errors. It is as if evolution chose systems in which parameters and structures allow simple results, in which parameters can control biological function robustly.
Of course, we only point the way. Additional compartments and additional structural complexity will surely be needed to deal with the workings of evolution. But these can be handled in a mathematically defined way, yielding approximate results with clear physical and biological interpretation. Combining the multi-domain model and membrane potential dependent conductance, one can model depolarization induced by extra potassium in lens [53, 55]
and cortical spreading depression (CSD) problem [74, 75, 71].
The ultimate goals will be (i) to provide as much precision in the mathematics and physics as we can, starting from first principles [62]; (ii) to provide a general basis for treatments of convection in other tissues that involve microcirculation. Computational models of these are not in hand, and may be hard to construct, since so little is know of those systems compared to the lens. With what we have learned here, we hope a general mathematical approach and model of the type we present here may be constructed and helpful in other systems with narrow extracellular spaces that are likely to need microcirculation to augment diffusion, like cardiac and skeletal muscle, kidney, liver, epithelia, and the extracellular space of the brain.
Author Contributions
Y.Z, S.X, and H.H did the model derivations and carried out the numerical simulations. R.S.E and H.H designed the study, coordinated the study, and commented on the manuscript. All authors gave final approval for publication.
Acknowledgments
This research is supported in part by the Fields Institute for Research in Mathematical Science (S.X., R.S.E., H.H.), the Natural Sciences and Engineering Research Council of Canada (H.H.).
References
References
-
[1]
R. S. Eisenberg, J. L. Rae,
Current-voltage
relationships in the crystalline lens, J Physiol 262 (2) (1976) 285–300.
URL http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1086902 - [2] R. Eisenberg, Electrical structure of biological cells and tissues: impedance spectroscopy, stereology, and singular perturbation theory, arXiv preprint arXiv:1511.01339.
- [3] B. R. Eisenberg, Skeletal muscle fibers: stereology applied to anisotropic and periodic structures 1 (1979) 274–284.
- [4] E. Barsoukov, J. R. Macdonald, Impedance spectroscopy: theory, experiment, and applications, John Wiley & Sons, 2018.
-
[5]
R. S. Eisenberg,
Structural
complexity, circuit models, and ion accumulation, Fed Proc 39 (5) (1980)
1540–3.
URL http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7364048 - [6] R. S. Eisenberg, Impedance measurement of the electrical structure of skeletal muscle (1983) 301–323.
-
[7]
R. S. Eisenberg, R. T. Mathias,
Structural
analysis of electrical properties of cells and tissues, CRC Crit Rev Bioeng
4 (3) (1980) 203–32.
URL http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=6256125 -
[8]
R. S. Eisenberg, R. T. Mathias, J. S. Rae,
Measurement,
modeling, and analysis of the linear electrical properties of cells, Ann N Y
Acad Sci 303 (1977) 342–54.
URL http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=290301 - [9] R. S. Eisenberg, Membranes and channels physiology and molecular biology (1984) 235–283.
-
[10]
R. T. Mathias, Analysis of
membrane properties using extrinsic noise (1984) 49–116doi:10.1007/978-1-4684-4850-4_3.
URL http://dx.doi.org/10.1007/978-1-4684-4850-4_3 -
[11]
L. Ebihara, R. T. Mathias,
Linear impedance studies of
voltage-dependent conductances in tissue cultured chick heart cells, Biophys
J 48 (3) (1985) 449–60.
doi:10.1016/S0006-3495(85)83800-5.
URL http://www.ncbi.nlm.nih.gov/pubmed/4041538 -
[12]
R. A. Levis, R. T. Mathias, R. S. Eisenberg,
Electrical
properties of sheep purkinje strands. electrical and chemical potentials in
the clefts, Biophys J 44 (2) (1983) 225–48.
URL http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=6360228 -
[13]
R. T. Mathias, L. Ebihara, M. Lieberman, E. A. Johnson,
Linear electrical
properties of passive and active currents in spherical heart cell clusters,
Biophys J 36 (1) (1981) 221–42.
doi:10.1016/S0006-3495(81)84725-X.
URL http://www.ncbi.nlm.nih.gov/pubmed/7284551 -
[14]
R. L. Milton, R. T. Mathias, R. S. Eisenberg,
Electrical
properties of the myotendon region of frog twitch muscle fibers measured in
the frequency domain, Biophys J 48 (2) (1985) 253–67.
URL http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=3876852 - [15] R. Valdiosera, C. Clausen, R. Eisenberg, Measurement of the impedance of frog skeletal muscle fibers, Biophys. J. 14 (1974) 295–314.
- [16] V. Barcilon, J. Cole, R. S. Eisenberg, A singular perturbation analysis of induced electric fields in nerve cells, SIAM J. Appl. Math. 21 (2) (1971) 339–354.
-
[17]
R. S. Eisenberg, V. Barcilon, R. T. Mathias,
Electrical
properties of spherical syncytia, Biophys J 25 (1) (1979) 151–80.
URL http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=262383 -
[18]
R. T. Mathias, J. L. Rae, R. S. Eisenberg,
Electrical
properties of structural components of the crystalline lens, Biophys J
25 (1) (1979) 181–201.
URL http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=262384 -
[19]
R. T. Mathias, J. L. Rae, R. S. Eisenberg,
The
lens as a nonuniform spherical syncytium, Biophys J 34 (1) (1981) 61–83.
URL http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7213932 -
[20]
J. L. Rae, R. T. Mathias, R. S. Eisenberg,
Physiological
role of the membranes and extracellular space with the ocular lens, Exp Eye
Res 35 (5) (1982) 471–89.
URL http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=6983449 -
[21]
R. T. Mathias, R. S. Eisenberg, R. Valdiosera,
Electrical
properties of frog skeletal muscle fibers interpreted with a mesh model of
the tubular system, Biophys J 17 (1) (1977) 57–93.
URL http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=831857 -
[22]
R. Valdiosera, C. Clausen, R. S. Eisenberg,
Impedance
of frog skeletal muscle fibers in various solutions, J Gen Physiol 63 (4)
(1974) 460–91.
URL http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=4544879 -
[23]
J. L. Rae, R. D. Thomson, R. S. Eisenberg,
The
effect of 2-4 dinitrophenol on cell to cell communication in the frog lens,
Exp Eye Res 35 (6) (1982) 597–609.
URL http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=6983973 -
[24]
G. J. Baldo, X. Gong, F. J. Martinez-Wittinghan, N. M. Kumar, N. B. Gilula,
R. T. Mathias, Gap
junctional coupling in lenses from alpha(8) connexin knockout mice, J Gen
Physiol 118 (5) (2001) 447–56.
URL http://www.ncbi.nlm.nih.gov/pubmed/11696604 -
[25]
P. J. Donaldson, L. S. Musil, R. T. Mathias,
Point: A critical
appraisal of the lens circulation model–an experimental paradigm for
understanding the maintenance of lens transparency?, Invest Ophthalmol Vis
Sci 51 (5) (2010) 2303–6.
doi:10.1167/iovs.10-5350.
URL http://www.ncbi.nlm.nih.gov/pubmed/20435604 -
[26]
P. Donaldson, J. Kistler, R. T. Mathias,
Molecular solutions to
mammalian lens transparency, News Physiol Sci 16 (2001) 118–23.
URL http://www.ncbi.nlm.nih.gov/pubmed/11443230 -
[27]
J. Gao, X. Sun, F. J. Martinez-Wittinghan, X. Gong, T. W. White, R. T. Mathias,
Connections between
connexins, calcium, and cataracts in the lens, J Gen Physiol 124 (4) (2004)
289–300.
doi:10.1085/jgp.200409121.
URL http://www.ncbi.nlm.nih.gov/pubmed/15452195 -
[28]
J. Gao, X. Sun, L. C. Moore, T. W. White, P. R. Brink, R. T. Mathias,
Lens intracellular
hydrostatic pressure is generated by the circulation of sodium and modulated
by gap junction coupling, J Gen Physiol 137 (6) (2011) 507–20.
doi:10.1085/jgp.201010538.
URL http://www.ncbi.nlm.nih.gov/pubmed/21624945 -
[29]
J. Gao, X. Sun, V. Yatsula, R. S. Wymore, R. T. Mathias,
Isoform-specific function
and distribution of na/k pumps in the frog lens epithelium, J Membr Biol
178 (2) (2000) 89–101.
URL http://www.ncbi.nlm.nih.gov/pubmed/11083898 -
[30]
R. T. Mathias, J. Kistler, P. Donaldson,
The lens circulation, J
Membr Biol 216 (1) (2007) 1–16.
doi:10.1007/s00232-007-9019-y.
URL http://www.ncbi.nlm.nih.gov/pubmed/17568975 -
[31]
R. T. Mathias, J. L. Rae,
Transport
properties of the lens, Am J Physiol 249 (3 Pt 1) (1985) C181–90.
URL http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2994483 -
[32]
R. T. Mathias, J. L. Rae,
The
lens: local transport and global transparency, Exp Eye Res 78 (3) (2004)
689–98.
URL http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15106948 -
[33]
R. T. Mathias, J. L. Rae, G. J. Baldo,
Physiological
properties of the normal lens, Physiol Rev 77 (1) (1997) 21–50.
URL http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9016299 -
[34]
R. T. Mathias, G. Riquelme, J. L. Rae,
Cell
to cell communication and ph in the frog lens, J Gen Physiol 98 (6) (1991)
1085–103.
URL http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1783895 -
[35]
R. T. Mathias, H. Wang,
Local osmosis and isotonic
transport, J Membr Biol 208 (1) (2005) 39–53.
doi:10.1007/s00232-005-0817-9.
URL http://www.ncbi.nlm.nih.gov/pubmed/16596445 -
[36]
R. T. Mathias, T. W. White, X. Gong,
Lens gap junctions in
growth, differentiation, and homeostasis, Physiol Rev 90 (1) (2010)
179–206.
doi:10.1152/physrev.00034.2009.
URL http://www.ncbi.nlm.nih.gov/pubmed/20086076 -
[37]
R. McNulty, H. Wang, R. T. Mathias, B. J. Ortwerth, R. J. Truscott,
S. Bassnett, Regulation of
tissue oxygen levels in the mammalian lens, J Physiol 559 (Pt 3) (2004)
883–98.
doi:10.1113/jphysiol.2004.068619.
URL http://www.ncbi.nlm.nih.gov/pubmed/15272034 -
[38]
J. L. Rae, C. Bartling, J. Rae, R. T. Mathias,
Dye
transfer between cells of the lens, J Membr Biol 150 (1) (1996) 89–103.
URL http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8699483 -
[39]
K. Varadaraj, S. Kumari, A. Shiels, R. T. Mathias,
Regulation of aquaporin
water permeability in the lens, Invest Ophthalmol Vis Sci 46 (4) (2005)
1393–402.
doi:10.1167/iovs.04-1217.
URL http://www.ncbi.nlm.nih.gov/pubmed/15790907 -
[40]
K. Varadaraj, C. Kushmerick, G. J. Baldo, S. Bassnett, A. Shiels, R. T.
Mathias, The role of mip
in lens fiber cell membrane transport, J Membr Biol 170 (3) (1999) 191–203.
URL http://www.ncbi.nlm.nih.gov/pubmed/10441663 -
[41]
E. Vaghefi, D. T. Malcolm, M. D. Jacobs, P. J. Donaldson,
Development of a 3d
finite element model of lens microcirculation, Biomed Eng Online 11 (2012)
69.
doi:10.1186/1475-925X-11-69.
URL https://www.ncbi.nlm.nih.gov/pubmed/22992294 -
[42]
E. Vaghefi, B. P. Pontre, M. D. Jacobs, P. J. Donaldson,
Visualizing ocular lens
fluid dynamics using mri: manipulation of steady state water content and
water fluxes, Am J Physiol Regul Integr Comp Physiol 301 (2) (2011)
R335–42.
doi:10.1152/ajpregu.00173.2011.
URL https://www.ncbi.nlm.nih.gov/pubmed/21593426 -
[43]
E. Vaghefi, N. Liu, P. J. Donaldson,
A computer model of lens
structure and function predicts experimental changes to steady state
properties and circulating currents, Biomed Eng Online 12 (2013) 85.
doi:10.1186/1475-925X-12-85.
URL https://www.ncbi.nlm.nih.gov/pubmed/23988187 - [44] H. D. Wu, P. J. Donaldson, E. Vaghefi, Review of the experimental background and implementation of computational models of the ocular lens microcirculation, IEEE Reviews in Biomedical Engineering 9 (2016) 163–176. doi:10.1109/RBME.2016.2583404.
-
[45]
P. J. Donaldson, Fluid in
equals fluid out–evidence for circulating fluid fluxes in the lens, Invest
Ophthalmol Vis Sci 53 (12).
doi:10.1167/iovs.12-11012.
URL https://www.ncbi.nlm.nih.gov/pubmed/23166303 -
[46]
P. J. Donaldson, A. C. Grey, B. Maceo Heilman, J. C. Lim, E. Vaghefi,
The physiological optics
of the lens, Prog Retin Eye Res 56 (2017) e1–e24.
doi:10.1016/j.preteyeres.2016.09.002.
URL https://www.ncbi.nlm.nih.gov/pubmed/27639549 -
[47]
J. Gao, X. Sun, L. C. Moore, P. R. Brink, T. W. White, R. T. Mathias,
The effect of size and
species on lens intracellular hydrostatic pressure, Invest Ophthalmol Vis
Sci 54 (1) (2013) 183–92.
doi:10.1167/iovs.12-10217.
URL http://www.ncbi.nlm.nih.gov/pubmed/23211824 -
[48]
K. L. Schey, R. S. Petrova, R. B. Gletten, P. J. Donaldson,
The role of aquaporins in
ocular lens homeostasis, Int J Mol Sci 18 (12).
doi:10.3390/ijms18122693.
URL https://www.ncbi.nlm.nih.gov/pubmed/29231874 -
[49]
E. Vaghefi, A. Kim, P. J. Donaldson,
Active maintenance of the
gradient of refractive index is required to sustain the optical properties of
the lens, Invest Ophthalmol Vis Sci 56 (12) (2015) 7195–208.
doi:10.1167/iovs.15-17861.
URL https://www.ncbi.nlm.nih.gov/pubmed/26540658 -
[50]
R. T. Mathias, Epithelial
water transport in a balanced gradient system, Biophys J 47 (6) (1985)
823–36.
doi:10.1016/S0006-3495(85)83986-2.
URL http://www.ncbi.nlm.nih.gov/pubmed/4016200 -
[51]
R. T. Mathias,
Steady-state
voltages, ion fluxes, and volume regulation in syncytial tissues 48 (3)
(1985) 435–448.
URL http://linkinghub.elsevier.com/retrieve/pii/S0006349585837991 - [52] G. Baldo, R. Mathias, Spatial variations in membrane properties in the intact rat lens, Biophysical journal 63 (2) (1992) 518–529.
- [53] N. Delamere, G. Duncan, A comparison of ion concentrations, potentials and conductances of amphibian, bovine and cephalopod lenses, The Journal of physiology 272 (1) (1977) 167–186.
- [54] R. T. Mathias, J. L. Rae, Steady state voltages in the frog lens, Current eye research 4 (4) (1985) 421–430.
- [55] D. T. K. Malcolm, A computational model of the ocular lens, Ph.D. thesis, ResearchSpace@ Auckland (2006).
- [56] I. G. Currie, I. Currie, Fundamental mechanics of fluids, Crc Press, 2002.
- [57] J. H. Ferziger, M. Peric, Computational methods for fluid dynamics, Springer Science & Business Media, 2012.
- [58] G. Duncan, K. Hightower, S. Gandolfi, J. Tomlinson, G. Maraini, Human lens membrane cation permeability increases with age., Investigative ophthalmology & visual science 30 (8) (1989) 1855–1859.
- [59] A. M. DeRosa, F. J. Martinez-Wittinghan, R. T. Mathias, T. W. White, Intercellular communication in lens development and disease, in: Gap junctions in development and disease, Springer, 2005, pp. 173–195.
- [60] A. Shiels, D. Mackay, A. Ionides, V. Berry, A. Moore, S. Bhattacharya, A missense mutation in the human connexin50 gene (gja8) underlies autosomal dominant “zonular pulverulent” cataract, on chromosome 1q, The American Journal of Human Genetics 62 (3) (1998) 526–532.
- [61] D. Mackay, A. Ionides, Z. Kibar, G. Rouleau, V. Berry, A. Moore, A. Shiels, S. Bhattacharya, Connexin46 mutations in autosomal dominant congenital cataract, The American Journal of Human Genetics 64 (5) (1999) 1357–1364.
- [62] S. Xu, B. Eisenberg, Z. Song, H. Huang, Osmosis through a semi-permeable membrane: a consistent approach to interactions, arXiv preprint arXiv:1806.00646.
- [63] J. Gao, X. Sun, T. W. White, N. A. Delamere, R. T. Mathias, Feedback regulation of intracellular hydrostatic pressure in surface cells of the lens, Biophysical journal 109 (9) (2015) 1830–1839.
- [64] O. R. Levine, R. B. Mellins, R. M. Senior, A. P. Fishman, The application of starling’s law of capillary exchange to the lungs, The Journal of clinical investigation 46 (6) (1967) 934–944.
- [65] B. Eisenberg, Life’s solutions are complex fluids. a mathematical challenge, arXiv preprint arXiv:1207.4737.
- [66] B. Eisenberg, Interacting ions in biophysics: real is not ideal, Biophysical journal 104 (9) (2013) 1849–1866.
- [67] L. Wan, S. Xu, M. Liao, C. Liu, P. Sheng, Self-consistent approach to global charge neutrality in electrokinetics: A surface potential trap model, Physical Review X 4 (1) (2014) 011042.
- [68] A. L. Hodgkin, A. F. Huxley, Currents carried by sodium and potassium ions through the membrane of the giant axon of loligo, The Journal of physiology 116 (4) (1952) 449–472.
- [69] A. L. Hodgkin, A. F. Huxley, The components of membrane conductance in the giant axon of loligo, The Journal of physiology 116 (4) (1952) 473–496.
- [70] Z. Song, X. Cao, H. Huang, Electroneutral models for dynamic poisson-nernst-planck systems, Physical Review E 97 (1) (2018) 012411.
-
[71]
Y. Mori,
A
multidomain model for ionic electrodiffusion and osmosis with an application
to cortical spreading depression, Physica D: Nonlinear Phenomena 308 (2015)
94 – 108.
doi:https://doi.org/10.1016/j.physd.2015.06.008.
URL http://www.sciencedirect.com/science/article/pii/S0167278915001086 - [72] C.-C. Lee, H. Lee, Y. Hyon, T.-C. Lin, C. Liu, New poisson–boltzmann type equations: one-dimensional solutions, Nonlinearity 24 (2) (2010) 431.
- [73] A. Peskoff, R. Eisenberg, Interpretation of some microelectrode measurements of electrical properties of cells, Annual review of biophysics and bioengineering 2 (1) (1973) 65–79.
-
[74]
J. C. Chang, K. C. Brennan, D. He, H. Huang, R. M. Miura, P. L. Wilson, J. J.
Wylie, A mathematical
model of the metabolic and perfusion effects on cortical spreading
depression, PLOS ONE 8 (2013) 1–9.
doi:10.1371/journal.pone.0070469.
URL https://doi.org/10.1371/journal.pone.0070469 -
[75]
W. Yao, H. Huang, R. M. Miura,
A continuum neuronal model
for the instigation and propagation of cortical spreading depression,
Bulletin of Mathematical Biology 73 (11) (2011) 2773–2790.
doi:10.1007/s11538-011-9647-3.
URL https://doi.org/10.1007/s11538-011-9647-3
Appendix A Model Parameters
Appendix B Dimensionless Parameters and Scales
The following dimensionless parameters’ value and scales calculation based on values in [55]
| Scales/Parameters | Value | Parameters | Value |
The can be find in the following equations.
Appendix C Non-dimensionalization
In this section, we derive the dimensionless model based on the lens, which has been widely studied. The major ions we considering here are sodium (), potassium () and chloride and the sodium-potassium pump which distributed on the surface of the lens. Although we restrict ourselves in this particular problem, the following procedure can be applied in a wide range of practical problems in biological syncytia.
C.1 Water circulation
In the following, we assume the typical length of lens is . The fluid system is driven by the osmotic gradient, which is generated by the sodium-potassium pump on the surface. In Eq. 7, the strength of sodium-potassium pump at surface depends on the ion’s concentration , which leads
| (53) |
where
| (54) |
We assume that the velocity at surface determines the characteristic velocity scale for the problem. We have ion fluxes in the intracellular, extracellular region in Eq. 5 and trans-membrane source of ion in Eq. 6 for ion .
At boundary of the intracellular space, due to the ion pump in Eq. 53 and assumption of conductance at surface that [51, 47], we have
| (55) |
Since inside of the lens, we obtain
| (56) |
This assumption obviously will have to be replaced in applications to other tissues, with a less particular distribution of channel proteins.
By the conservation of fluxes for each ion in Eq. 4, we get
| (57) |
where . Therefore, Eq. 55 becomes
| (58) |
Adding up all three fluxes in Eq. 58 and since in the extracellular region each ion diffusion coefficient are at the same level of approximation, i.e.
| (59) |
and based on Eq. 10 , we get
| (60) |
The strength of the ion pump depends on the ion concentration in Eq. 54 . We choose the scale of is based on an experimental estimation [51]. Using Eq. 60, we take the scale for and to be and as
| (61) |
By mass conservation expressed in Eq. 1, we naturally get the scale of as
| (62) |
Furthermore, is used for the scale of electric potential and . For the extracellular velocity in Eq. 2, we have
| (63) |
We think the term balance the velocity . The scale for extracellular pressure is then choose
Therefore, we get
| (64) |
where . For the intracellular velocity, we have
| (65) |
We claim term and balance at the same level. Therefore, we choose the same scale for the intracellular and extracellular pressure, namely,
Then Eq. 65 becomes
| (66) |
where
In all, the fluid system Eq. 1 becomes
| (67) |
with boundary condition
where
C.2 Ions circulation
The velocity scales and diffusion coefficients in the extracellular and intracellular space are at different levels of approximation in our approach. In the following, we put the characteristic diffusion coefficients at intracellular and extracellular region and scale of concentration as
In this way, we get Peclet number in the extracellular and intracellular and dimensionless Nernst potential as
Because inside of lens, we have system as in Mathias’s model [51],
| (68) |
with boundary condition
and system as
| (69) |
with boundary condition
where
The concentration of can be solved from the following equations
| (70) |
where
| (71) |
From Eq. 11 and use the fact and assumption that and , we have
| (72) |
with boundary condition
| (73) |
where
and
Appendix D Effect of permeability