figurec
]http://udel.edu/ absingh/
A mechanistic first–passage time framework for bacterial cell-division timing
Abstract
How exponentially growing cells maintain size homeostasis is an important fundamental problem. Recent single-cell studies in prokaryotes have uncovered the adder principle, where cells on average, add a fixed size (volume) from birth to division. Interestingly, this added volume differs considerably among genetically-identical newborn cells with similar sizes suggesting a stochastic component in the timing of cell-division. To mechanistically explain the adder principle, we consider a time-keeper protein that begins to get stochastically expressed after cell birth at a rate proportional to the volume. Cell-division time is formulated as the first-passage time for protein copy numbers to hit a fixed threshold. Consistent with data, the model predicts that while the mean cell-division time decreases with increasing size of newborns, the noise in timing increases with size at birth. Intriguingly, our results show that the distribution of the volume added between successive cell-division events is independent of the newborn cell size. This was dramatically seen in experimental studies, where histograms of the added volume corresponding to different newborn sizes collapsed on top of each other. The model provides further insights consistent with experimental observations: the distributions of the added volume and the cell-division time when scaled by their respective means become invariant of the growth rate. Finally, we discuss various modifications to the proposed model that lead to deviations from the adder principle. In summary, our simple yet elegant model explains key experimental findings and suggests a mechanism for regulating both the mean and fluctuations in cell-division timing for size control.
pacs:
Introduction
One common theme underlying life across all organisms is recurring cycles of growth of a cell, and its subsequent division into two viable progenies. How an isogenic population of proliferating cells maintains a narrow distribution of cell size, a property known as cell size homeostasis, has been a topic of research for long time yfk75 ; fan77 ; wrp10 ; tes12a ; mys12 ; am14 ; iwh14 ; rhk14 ; csk14 ; srb14 ; onl14 ; tbs15 ; jut15 ; dvx15 ; ro15 . Over the years, a number of theories have been proposed to explain cell size control from a phenomenological viewpoint. Recent single-cell experiments have shown that several prokaryotes such as Escherichia coli, Caulobacter crescentus, Bacillus subtilis, Pseudomonas aeruginosa, and Desulfovibrio vulgaris Hildenborough csk14 ; tbs15 ; dvx15 ; fdm15 follow what is called an adder principle. The adder model states that a cell, on average, adds a constant size between birth and division, irrespective of its size at birth vk97 ; iwh14 ; am14 ; csk14 ; srb14 ; jut15 ; tbs15 ; dvx15 . The adder mechanism has also been found to be present in the G1 phase of budding yeast Saccharomyces cerevisiae srb14 , suggesting that it is possibly employed by a wide range of organisms.
Despite a significant development in the understanding at phenomenological level, the molecular mechanisms underpinning the cell size control are not well understood DoB03 ; ro15 . A prevalent class of models posits that a protein acts as a time-keeper between subsequent occurrences of an important event in the cell cycle tcd74 ; fan75 ; onl14 ; dvx15 ; bzd15 ; do68 ; sm73 ; am14 ; ha15 . This protein is synthesized at a rate proportional to instantaneous volume (size) and the event of interest, which could either be the division itself tcd74 ; fan75 ; onl14 ; dvx15 ; bzd15 or commitment to division after a constant time do68 ; sm73 ; am14 ; ha15 , takes place when the protein reaches a certain threshold. It has been previously shown that this simple biophysical mechanism can lead to the adder principle of cell size control in the mean sense am14 ; ha15 ; bzd15 . Interestingly, recent experimental data on bacteria has quite fascinating stochastic component as well. For instance, not only the mean but the distribution of the volume added between two division events itself is independent of the volume at birth for a given growth condition. Also, in different growth conditions, the distributions of the added volume collapse if scaled by their respective means tbs15 . It remains to be seen whether these stochastic traits can be produced by the aforementioned molecular mechanism.
A plausible source of the stochasticity is the noise in gene expression wherein randomness in transcription and translation results in significant cell to cell variation in protein levels bkc03 ; rao05 ; rav08 ; keb05 ; sis13 . Here, we consider a time-keeper protein between two consecutive division events and show that its probabilistic expression can indeed manifest as the stochastic component in cell size. An overview of the mechanism and it leading to added volume distribution independent of the initial cell volume are depicted in Fig. 2. Considering a cell of given volume at birth, we assume that its volume grows exponentially until the time at which the time-keeper protein triggers the division. The production of the protein is started right after the birth of a cell. The production rate of the protein scales with the volume, i.e., increases exponentially (Fig. 2(a)). Cell division time is modeled as the first-passage time for protein copy numbers to attain a certain threshold (Fig. 2(b)). The distribution of the first-passage time is then used to compute the distribution of the volume added between birth to division (Fig. 2(c),(d)). Our results further show that distributions of the added volume, and cell division time have scale-invariant forms: they collapse upon rescaling with their respective means in different growth conditions. Lastly, we discuss implications of these findings in identification of the time-keeper protein. We also deliberate upon various modifications to the proposed model that result in deviations from the adder principle.
Model description
Considering a cell with volume at birth , its volume at a time after birth is given by
| (1) |
where represents cell growth rate. As shown in Fig. 2(a), production of a protein is started right after the birth of a cell. A stochastic gene expression model describing the time evolution of this protein is described below.
Let denote the protein count at time . Assuming constitutive transcription and mRNA half life is smaller than the cell cycle time, we make the translation burst approximation where each mRNA molecule degrades instantaneously after producing a burst of protein molecules Pau05 ; FCX06 ; ShS08 ; Ber78 ; EJF11 ; Rig79 . Protein synthesis is given by the following model:
| (2) |
The variable denotes the size of protein burst which, for each , is independently drawn from a positive-valued distribution. It essentially represents the number of protein molecules synthesized in a single mRNA lifetime and typically follows a geometric distribution Ber78 ; Rig79 ; FCX06 ; SRC10 ; SiB12 ; SRD12 . Furthermore, the production rate of the protein or alternatively the burst arrival rate (transcription rate) is assumed to be volume dependent. More specifically, we consider as
| (3) |
where is a proportionality constant. In this formulation, the production rate scaling with the cell volume is an essential component of maintaining concentration homeostasis. Indeed such a dependency of transcription rate on cell volume has been observed in mammalian cells pnb15 .
Note that the protein count
| (4) |
is a sum of independent and identically distributed random variables ’s, where is the number of bursts arrived (transcription events) in time interval . The key assumption is that the cell divides when the protein level crosses a threshold . We would like to mention that though the model described here is for gene expression, it can be used to model any process involving accumulation of molecules wherein the production rate scales with the volume. A general distribution for allows a wider range of processes to be covered. The scope can be further widened by considering the parameters , , and to be functions of the growth rate . In the next section, we quantify the cell division time by modeling it as the first time taken by to cross .
Cell division time as a first-passage time problem
The first-passage time () for the stochastic process to cross a threshold is mathematically defined as:
| (5) |
For the stochastic gene expression model described in the previous section, time evolution of and corresponding first-passage times for cells with different initial volumes is depicted in Fig. 2(b). To characterize the distribution of , we need to quantify two quantities: arrival time of a burst, and minimum number of burst (transcription) events required for to cross . The conditional probability density function of for a given cell volume at birth is then given as
| (6) |
where represents the probability density function of , and represents the probability mass function of .
Since can only increase in time, the distribution of can be determined by the distribution of the burst size using the relation:
| (7) |
As an specific yet physiologically relevant example, when the burst size distribution is considered to be geometric, is given by
| (8) |
where represents the mean (or expected value) of burst size GhS14 ; SiD14 . Furthermore, the probability density function of arrival event is governed by the underlying burst arrival process. In our case, the transcription (burst arrival) rate is time dependent. Therefore, the burst arrival process is an inhomogeneous Poisson process for which the probability density function of the arrival times are available in literature (see Bax82 ; PSZ00 ). In particular, we have
| (9) |
The transcription or burst arrival rate is referred to as the intensity function of the corresponding inhomogeneous Poisson process. Also, is the mean value function of the inhomogeneous poisson process
| (10) |
Using (9) in (6), the expression for the probability density function of for a cell of given volume at birth becomes
| (11) |
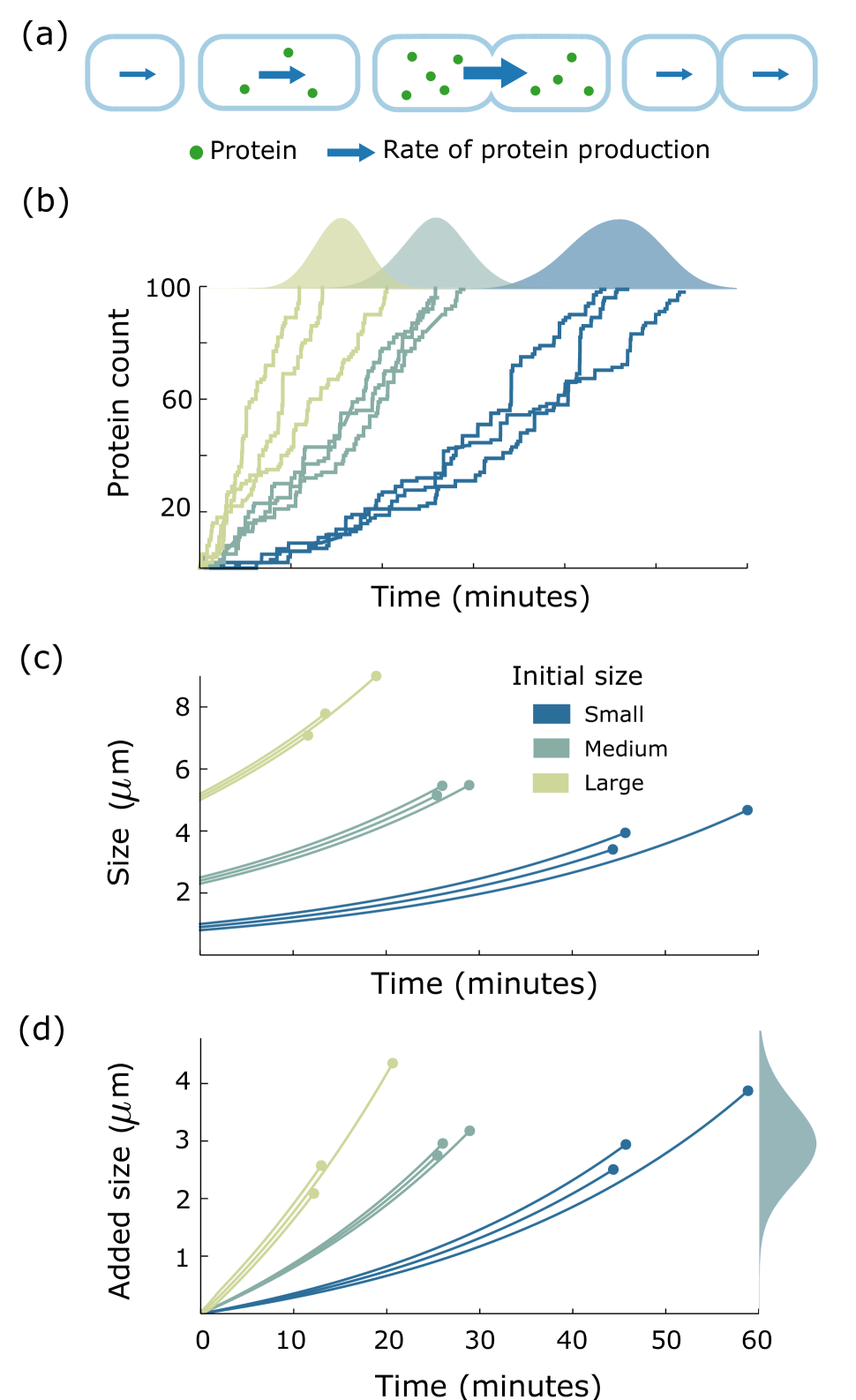
The conditional distribution in (11) qualitatively emulates the experimental observations in tbs15 that the mean cell division time decreases as the cell size at birth is increased (refer to SI, section S1). This is intuitively expected as a cell with large size a birth will have a higher transcription rate as compared to a cell with small size. Hence, on average, the time taken by the protein to reach the prescribed threshold is smaller in the larger cell. The model also predicts that the noise (quantified using coefficient of variation squared, ) in the cell division time increases as new born cell volume is increased (see Fig. 3 (left)). This prediction is consistent with data from tbs15 , as shown on the right part of Fig. 3 . The noise behavior can be explained by observing that on average a cell with smaller volume at birth takes more time for division. Therefore the fluctuations are time averaged, leading to a smaller noise in division time as compared to that of a cell with larger volume at birth.

Distribution of the volume added between divisions
In the previous section, we determined the distribution of the division time for a cell of given initial volume . Coupling this with the fact that volume of the cell grows exponentially in time, we can find the distribution of the volume added to . More precisely, the volume added from birth to division (denoted by ) is given as
| (12) |
The distribution given in (11) can be used to find the distribution of (see SI, section S2). Let denote the probability density function of , then we have
| (13) |
One striking observation is that is independent of the initial volume (see Fig 2 for a depiction of this). This is in agreement with the experimental observations that the distribution of the added volume does not depend on the size of the cell at birth iwh14 ; tbs15 . Further, we can also use the expression in (13) to find moments of . We will first discuss the expression of mean , particularly emphasizing its dependence on the growth rate. The higher order moments are taken up next.
Mean of added volume
The distribution of given in (13) is an Erlang distribution conditioned on , the minimum number of burst events required for the protein to cross the threshold. The formula for can be written as
| (14) |
where we have assumed that protein is produced in geometric bursts with mean burst size (see SI, sections S1, S2). It can be seen that the if we consider the parameters , , and to be independent of the growth rate , average volume added is a linearly increasing function of . This is consistent with the analysis and experimental data on Pseudomonas aeruginosa dvx15 . In contrast, other studies have mentioned that there is an exponential relationship between and instead csk14 ; srb14 ; tbs15 . This nebulous aspect of the data has been attributed to narrow range of achievable growth rates which makes it difficult to discern a linear dependency from an exponential one dvx15 . While suitable forms of , , and as functions of can generate a desired growth rate dependency of the added volume, we discuss a modification in the model to see an exponential relationship between them for the case when , , and are constants.
Let us consider that instead of accounting for the time between birth to division, the protein accounts for time between other two events in the cell cycle. More specifically, we consider initiation of DNA replication takes place when sufficient time-keeper protein has been accumulated per origin of replication do68 ; am14 ; ha15 . The corresponding division event is assumed to occur with a constant delay of after an initiation. Here the delay is what is called period whereby represents the time to replicate the DNA and denotes the time between DNA replication and division CoH68 ; Coo12 . As shown in ha15 (section 3.2), the volume added between two consecutive initiation events for each origin of replication is same as in our previous model. Further, the average volume added between divisions associated associated with these initiations (denoted by ) is related with as
| (15) |
When , , and are constants, the expression in (15) suggests two different regimes of how depends upon . For small values of , we have . Therefore the mean added volume increases linearly with the growth rate. For large enough values of , the exponential term starts dominating which leads to exponential dependence of the average volume added with change in the growth rate. This also means that if the growth rate is small, it is not possible to distinguish whether the underlying mechanism accounts for volume added between two division events or two initiation events as the data will show linear dependence of the average added volume with changes in dvx15 . Notice that a pure exponential relationship between and can also be obtained by having a linearly increasing function of . For this particular case, the volume accounted for by each origin of replication will become invariant of the growth rate as suggested in ha15 ; tah15 .
To sum up, the case when the protein accounts for volume added between two divisions gives a linear dependency of the mean added volume on growth rate. However, an exponential dependence between these quantities can be achieved by having the protein account for volume added between two initiation events. We now go back to discussing the higher order moments of the division to division model.
Higher order moments of added volume and scale invariance of distributions
We can use the distribution of to get its higher order statistics (see SI, section S2). In particular when the protein production is considered in geometric bursts, the coefficient of variation squared () and skewness () are given by
| (16) |
These formulas show that both and skewness do not depend on the growth rate . It turns out an even more general property is true: an appropriately scaled order moment of , i.e., is independent . This arises from the fact that the distribution of can be written in the following form
| (17) |
regardless of the distribution of the burst size . An important implication of above form of distribution of is the scale invariance property: the shape of the distribution across different growth rates is essentially same, and a single parameter is sufficient to characterize the distribution of gac13 . Recent experimental data have also exhibited the scale invariance property tbs15 ; koj14 ; bzd15 .
Interestingly, the above invariance property is not limited to the distribution of the added volume . Ignoring the partitioning errors in the volume, it can be seen that in steady-state the cell-size distribution at birth is approximately same as the distribution of tbs15 . Also, the size at division is . Thus, the scale invariance of immediately implies scale invariance of the distributions of cell sizes at birth and division tbs15 . Moreover, the distribution of the division time can also be determined by unconditioning (11) with respect to the distribution of the initial volume . As shown in the SI (section S3), the distribution of the division time also has the scale invariance property which is in agreement with the results in ics14 ; iwh14 .
Discussion
In this work, we studied a molecular mechanism that can lead to adder principle of cell size control. This mechanism imagines a protein sensing the volume added between birth to division tcd74 ; fan75 ; onl14 ; dvx15 ; bzd15 or two other events in the cell cycle do68 ; sm73 ; am14 ; ha15 . Our work shows that this mechanism can exhibit the stochastic traits observed in the data tbs15 . In particular, it is shown that the distribution of volume added between birth to division is independent of the initial cell volume tbs15 . Further, the distributions of key quantities such as the added volume, division time, volume at birth, volume at division, etc. show the scale invariance property tbs15 . Our study also revealed that the noise in division time increases with increase in cell size at birth, which was validated from the available data from tbs15 . Here, we discuss the implications of these results.
Potential candidates for the time-keeper protein
Among many proteins involved in the process, prominent candidates for the time-keeper are FtsZ and DnaA. More specifically, if the constant volume addition is considered between division to division, FtsZ is a potential candidate for the protein bil90 ; lut07 ; eao10 ; deb10 ; ro15 . It plays an important role in determination of the timing of cell division which is triggered upon assembly of FtsZ into a ring structure ade09 . It has been proposed that the accumulation of FtsZ up to a critical level is required for cell division bil90 ; chl12 . Likewise, the protein DnaA is known to regulate the timing of initiation of replication, thus presenting a strong candidature for the protein if the constant volume is added between two initiation events alh87 ; lsh89 ; ro15 . In this case, initiation is thought to occur when a critical number of DnaA-ATP molecules are available DoB03 . Upon initiation, these DnaA-ATP molecules get deactivated by converting to DnaA-ADP sbk87 ; DoB03 . While it is not clear yet whether the production of DnaA or its conversion to DnaA-ATP is a rate limiting step in the initiation process, the model presented here can account for both cases as long as the conversion to DnaA-ATP happens at a volume dependent rate.
We can employ the closed-form expressions for the moments of developed in this work to investigate roles of these candidate proteins. Considering geometrically distributed burst of proteins, the expressions of mean and coefficient of variation squared () are given by (14) and (16) respectively. Thus, increasing the threshold or decreasing the mean burst size of the time-keeper protein should result in decrease in the of the added volume. Experimentally, the mean burst size can be altered by changing the translation rate of the proteins using techniques such as mutations in the Shine-Dalgarno sequence. Changing the threshold can be achieved by changing the protein sequence which affects its function and thus leads to a different number of protein molecules being required for division. It is important to point out cell-size control can possibly have mechanisms in place to overrule such tweaking. One possible way to overcome this could be to appropriately change the transcription rate scaling factor by promoter mutations, along with changes in or such that the added volume is same in the mean-sense.
We also acknowledge that a complex process like cell division may have a lot more going on than a simple protein carrying out the size and time control. For instance, there is some evidence of DnaA not being solely responsible for the timing of initiation fft15 , a cell compensating for a larger or smaller initiation time by adjusting the genome replication time bsk96 ; hkc12 ; chl12 , etc. Along the same lines, FtsZ ring formation is inhibited upon DNA damage which suggests that a viable copy of DNA is required for division to proceed mcl98 ; mhl11 . It appears that several key proteins follow the dynamics of the hypothetical protein we considered and at important stages, check points are established for proper coordination. Nonetheless, our model can shed light into how perturbations in expression of these proteins can lead to experimentally observable changes. This provides exciting avenues for investigating different candidate proteins by examining the effect of alterations in gene expression parameters.
Other sources of noise
The source of noise accounted for in this work is the intrinstic noise arising because of random birth events of mRNA/protein molecules, and death of mRNA molecules. In principle, there are other sources of noise such as cell-to-cell variation in cell specific factors such as enzyme levels which could affect the expression of the time-keeper protein and, in turn, influence the distributions of division time, cell size, etc. It is also possible that noise arising out of other factors dominates the noise from stochastic expression.
One important parameter in our model is the event threshold which we have assumed to be fixed. It is possible that instead of a strict requirement of exactly molecules, the division event has an increased propensity as the protein count gets closer to . Our analysis in SI, section 4 shows that in order to get independent of the cell volume at birth , the propensity of division requires a strict attainment of molecules. Thus, the alternate mechanism can be ruled out.
Recall our discussion in prevision section that decreases as the threshold is increased. Thus for a very large threshold, the contribution from expression of the protein is negligible. To get a of without other factors being counted in, we need a threshold of about molecules. Interestingly, the number of DnaA-ATP molecules required for initiation are around DoB03 . The threshold for FtsZ, however, is thought to be somewhere between molecules rvm03 to molecules lse98 . Therefore the stochastic expression of protein suffices to account for noise in if the initiation to initiation mechanism via DnaA is the key regulator of cell cycle. However, it becomes inevitable that other sources of noise are also considered in the model if the regulation is from division to division via FtsZ. One possibility is to consider the cell-to-cell variations in the growth rate. Likewise, in the case when the protein accounts for the volume added between two initiation events, partitioning errors can be introduced upon division of a cell. Accounting for these factors would provide a better insight into the process.
Deviations from the adder principle
Recently it has been proposed that cells employ a generalized version of the adder principle wherein the volume added between divisions depends upon the cell volume at birth jut15 ; tpp15 . An important implication of this is on the time a cell will take to converge to steady-state value. For example if the added volume decreases with then a large cell will converge faster than it would have in a perfect adder strategy.
There could be several ways to get deviations from the adder principle. For instance, if we consider that the time-keeper protein does not degrade fully upon division and the remaining proteins are divided in the daughter cells in proportion to their respective volumes at birth, the added volume decreases as volume of daughter cell is increased. This is because if there are already time-keeper proteins present in the cell at the time of its birth, the threshold will be achieved earlier than the case when there were no proteins at birth. As a result, the added volume will be smaller as compared to an adder principle. Another possible way of getting such deviation could be if the mean burst size is an increasing function of the cell volume. It could also be explained by similar reasoning that it leads to a smaller time to reach the copy number threshold of the protein. In contrast, to get a higher added volume for a increase in , we can curb the scaling of protein accumulation with the cell volume. One example is to assume a transcription rate of the form where the transcription rate saturates with increase in volume. Alternatively, this effect could also be achieved by considering that instead of cell division occurring upon achieving a constant volume addition, its propensity increases as the added volume increases.
Summary
This paper shows that the stochastic accumulation of a time-keeper protein can lead to adder principle of cell size control. We derived analytical formulas for the division time and volume added between birth to division. These expressions were used to show that the volume added is independent of the cell size at birth, consistent with experimental data. Furthermore, the distributions of added volume and division time also show scale invariance property wherein the distribution can be uniquely determined by its mean in a given growth condition. We also discussed the implications of these results in identifying a possible molecular mechanism underlying the cell-size control. Finally, we discussed how the proposed mechanism can be modified to get more general behaviors. Future work will involve accounting for other sources of noise such as growth rate fluctuations, partitioning errors, etc.
Supplementary Information
S1 Remarks on the conditional distribution of given cell volume at birth
As discussed in (11) in the main text, the probability density function of the first-passage time given cell volume at birth is given by
| (S1.1) |
Here, represents the probability mass function of (the minimum number of transcription events required for protein level to cross a threshold ). The relation in (7) can be used to quantify the distribution from the distribution of protein burst size . We present the form of distribution of for two relevant cases here: when protein burst size is one with probability one, and when the protein burst size is geometric Ber78 ; Rig79 ; FCX06 ; SRC10 ; SiB12 ; SRD12 .
When the burst size is one with probability one, exactly events are required for the protein level to reach for the first time. That is, we have
| (S1.2) |
where is the Kronecker’s delta which is one when and zero otherwise.
For the case where the burst size follows a geometric distribution Ber78 ; Rig79 ; FCX06 ; SRC10 ; SiB12 ; SRD12 , the calculation of the minimum number of transcription events for this distribution has been previously done in our works GhS14 ; SiD14 . The probability mass function of is given by
| (S1.3) |
Here represents the mean protein burst size. Further, the first three statistical moments of given by the above probability mass function are
| (S1.4) | |||
| (S1.5) | |||
| (S1.6) |
Mean FPT given newborn size
The expression of probability density function in (S1.1) can be used to determine the mean numerically. As we mentioned in the main text that, consistent with experiments, the mean decreases with increase in the volume of the cell at birth (). That is, a large cell on average divides earlier than a small cell. This is depicted in Fig. S1.1.
S2 Distribution of
Let us assume that the initial volume of a (newborn) cell is . Our primary assumption is that the cell divides at the first-passage time we computed in the previous section. Representing the volume added to the cell’s volume at birth until the event takes place by , we have
| (S2.1) |
We already have the distribution of in (S1.1). We use it to determine the distribution of as follows.
| (S2.2) | ||||
| (S2.3) | ||||
| (S2.4) |
Therefore the probability density function of is given by
| (S2.5) | ||||
| (S2.6) | ||||
| (S2.7) |
Note that . Also
Hence we can write the probability density of as
| (S2.8) | ||||
| (S2.9) |
Notice that this distribution is an Erlang distribution conditioned to the random variable .
Moments of
Mean
Since the distribution of is conditional Erlang, we have the following expression for mean .
| (S2.10) |
Second order moment
The second order moment of is given by
| (S2.11) |
Calculation of third order moment
The third order moment of is given by
| (S2.12) |
When the burst size is one with probability one, we have with probability one. The formulas of mean, and skewness of simplify to
| (S2.13) |
When the burst size is geometric, we get the following expressions:
| (S2.14) | |||
| (S2.15) | |||
| (S2.16) |
We also note that that the skewness of is positive in both cases considered above which is consistent with previous results vk97 .
Scale invariance of the distribution of
It has been shown in tbs15 that the distributions of the added volume in different growth conditions collapse when rescaled by respective . Mathematically, we want to show that the probability density function has the following form (tbs15, , Supplementary Information equation 36):
| (S2.17) |
where is an arbitrary normalized function. For the distribution in (S2.9), let us consider the following function for
| (S2.18) |
where is the expected value of the minimum number of transcription events required for the protein to cross the threshold and is given by . Also, it is related with as described in (S2.10).
For the function given in (S2.18), we have
| (S2.19) | ||||
| (S2.20) | ||||
| (S2.21) |
This establishes the scale invariance of the distribution .
One consequence of the scale invariance property of is that the normalized moments are independent of the growth conditions (tbs15, , Supplementary Information). This can be checked as follows.
The order conditional moment of (conditioned with respect to ) is given by order moment of an Erlang distribution. Thus
| (S2.22) | ||||
| (S2.23) |
Therefore using (S2.10), we have
| (S2.24) |
which is independent of the growth rate.
This fact can be used to show that statistical measures such as noise () and skewness are independent of the growth rate. Take for instance. It is defined as
| (S2.25) |
By the scale invariance property, is independent of the growth rate . Thus, the noise is also independent of . Similarly, skewness is given by
| (S2.26) | ||||
| (S2.27) |
which is again independent of by the scale invariance property.
S3 Distribution of FPT
FPT distribution can be obtained from (Equation S1.10) by solving
| (S3.1) |
where is the probability distribution of cell volumes at birth. Ignoring the errors in partitioning of volume, it can be seen that tbs15 . Using this relation, the FPT distribution is given by
| (S3.2) | ||||
| (S3.3) | ||||
| (S3.6) |
FPT moments can be written as
| (S3.9) | ||||
| (S3.10) |
The normalized moments are
| (S3.11) |
This implies, as shown for , that the noise, skewness and higher order moments are indendent of growth rate. Furthermore, if we use the function
| (S3.14) |
then FPT distribution can be written as
| (S3.15) |
Thus, the FPT distribution is also scale invariant. In tbs15 constant is equal to . We found that for different distributions of burst size and several values of threshold .
S4 Theoretical constraints on the molecular mechanism underlying adder principle
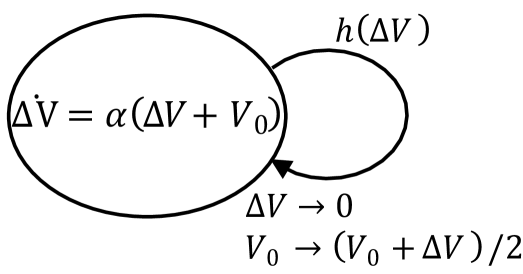
Let us consider a phenomenological description of the adder principle in terms of a hybrid system as shown in Fig. S4.1. Assuming the volume of a cell at birth as , the added volume follows a deterministic dynamics as . The hazard rate for division event is assumed to be some general function . The adder principle states that the division occurs, on average, when a constant volume has been added, irrespective of the initial cell volume .
Using the Dynkin’s formula dav93 , the expected value of the added volume follows
| (S4.1) | ||||
| (S4.2) |
We have made the mean-field approximation in the second equation above. In steady state, a solution which has independent of is only possible if the hazard rate is a dirac delta function . Therefore any mechanism which actively senses the added volume has to trigger the division the moment it reaches the prescribed threshold. The time-keeper protein based mechanism proposed in this work satisfied this necessary condition, and since it produced the added model in distribution sense as well, it shows that this theoretical constraint is both necessary and sufficient.
S5 Additional information on Fig. 2 in the main text
To study the effect of volume at birth on the noise in generation time () , we selected the rescaled mean and standard deviation of the generation time given the smallest and the largest volumes at birth from Fig. 2D in tbs15 . We assumed that the generation time has a normal distribution with mean and standard deviation given by the volume at birth (small and large). We drew 150 generation time samples for each volume at birth from the assumed distribution (150 is the number of samples per point used to generate plot in Fig. 2D). Using bootstrapping we tested if the of the small cells is larger than the of large cells. The p value obtained is . The 95% confidence interval is given in the following table.
| Lower bound | Median | Upper bound | |
| Small volume | 0.0424 | 0.0545 | 0.0686 |
| Large volume | 0.0796 | 0.1030 | 0.1313 |
Acknowledgements.
AS is supported by the National Science Foundation Grant DMS-1312926. The authors thank Prof. Suckjoon Jun for providing experimental data to compare with model predictions on noise in division time (Fig. 2).References
References
- [1] A. Yen, J. Fried, T. Kitahara, Annabel Strife, and B.D. Clarkson. The kinetic significance of cell size. Experimental Cell Research, 95:303 – 310, 1975.
- [2] PA Fantes. Control of cell size and cycle time in Schizosaccharomyces pombe. Journal of Cell Science, 24:51–67, 1977.
- [3] Ping Wang, Lydia Robert, James Pelletier, Wei Lien Dang, Francois Taddei, Andrew Wright, and Suckjoon Jun. Robust growth of Escherichia coli. Current Biology, 20:1099–1103, 2010.
- [4] Jonathan J. Turner, Jennifer C. Ewald, and Jan M. Skotheim. Cell size control in yeast. Current Biology, 22:R350–R359, 2012.
- [5] Wallace F. Marshall, Kevin D. Young, Matthew Swaffer, Elizabeth Wood, Paul Nurse, Akatsuki Kimura, Joseph Frankel, John Wallingford, Virginia Walbot, Xian Qu, and Adrienne HK Roeder. What determines cell size? BMC Biology, 10:101, 2012.
- [6] Ariel Amir. Cell size regulation in bacteria. Physical Review Letters, 112:208102, 2014.
- [7] Srividya Iyer-Biswas, Charles S. Wright, Jonathan T. Henry, Klevin Lo, Stanislav Burov, Yihan Lin, Gavin E. Crooks, Sean Crosson, Aaron R. Dinner, and Norbert F. Scherer. Scaling laws governing stochastic growth and division of single bacterial cells. Proceedings of the National Academy of Sciences, 111:15912–15917, 2014.
- [8] Lydia Robert, Marc Hoffmann, Nathalie Krell, Stéphane Aymerich, Jérôme Robert, and Marie Doumic. Division in Escherichia coli is triggered by a size-sensing rather than a timing mechanism. BMC Biology, 12:1433–1446, 2014.
- [9] Manuel Campos, Ivan V. Surovtsev, Setsu Kato, Ahmad Paintdakhi, Bruno Beltran, Sarah E. Ebmeier, and Christine Jacobs-Wagner. A constant size extension drives bacterial cell size homeostasis. Cell, 159:1433–1446, 2014.
- [10] Ilya Soifer, Lydia Robert, Naama Barkai, and Ariel Amir. Single-cell analysis of growth in budding yeast and bacteria reveals a common size regulation strategy. arXiv preprint arXiv:1410.4771, 2014.
- [11] Matteo Osella, Eileen Nugent, and Marco Cosentino Lagomarsino. Concerted control of Escherichia coli cell division. Proceedings of the National Academy of Sciences, 111:3431–3435, 2014.
- [12] Sattar Taheri-Araghi, Serena Bradde, John T. Sauls, Norbert S. Hill, Petra Anne Levin, Johan Paulsson, Massimo Vergassola, and Suckjoon Jun. Cell-size control and homeostasis in bacteria. Current Biology, 25:385–391, 2015.
- [13] Suckjoon Jun and Sattar Taheri-Araghi. Cell-size maintenance: universal strategy revealed. Trends in Microbiology, 23:4–6, 2015.
- [14] Maxime Deforet, Dave van Ditmarsch, and João B. Xavier. Cell-size homeostasis and the incremental rule in a bacterial pathogen. Biophysical Journal, 109:521–528, 2015.
- [15] Lydia Robert. Size sensors in bacteria, cell cycle control, and size control. Frontiers in Microbiology, 6:515, 2015.
- [16] Anouchka Fievet, Adrien Ducret, Tâm Mignot, Odile Valette, Lydia Robert, Romain Pardoux, Alain Roger Dolla, and Corinne Aubert. Single-cell analysis of growth and cell division of the anaerobe Desulfovibrio vulgaris Hildenborough. Frontiers in Microbiology, 6:1378, 2015.
- [17] W. J. Voorn and L. J. H. Koppes. Skew or third moment of bacterial generation times. Archives of Microbiology, 169:43–51, 1997.
- [18] William D Donachie and Garry W Blakely. Coupling the initiation of chromosome replication to cell size in Escherichia coli. Current Opinion in Microbiology, 6:146–150, 2003.
- [19] RM Teather, JF Collins, and WD Donachie. Quantal behavior of a diffusible factor which initiates septum formation at potential division sites in Escherichia coli. Journal of Bacteriology, 118:407–413, 1974.
- [20] PA Fantes, WD Grant, RH Pritchard, PE Sudbery, and AE Wheals. The regulation of cell size and the control of mitosis. Journal of Theoretical Biology, 50:213–244, 1975.
- [21] Markus Basan, Manlu Zhu, Xiongfeng Dai, Mya Warren, Daniel Sévin, Yi-Ping Wang, and Terence Hwa. Inflating bacterial cells by increased protein synthesis. Molecular Systems Biology, 11:836, 2015.
- [22] W. D. Donachie. Relationship between cell size and time of initiation of DNA replication. Nature, 219:1077–1079, 1968.
- [23] L. Sompayrac and O. Maaløe. Autorepressor model for control of DNA replication. Nature, 241:133–135, 1973.
- [24] Po-Yi Ho and Ariel Amir. Simultaneous regulation of cell size and chromosome replication in bacteria. Microbial Physiology and Metabolism, 6:662, 2015.
- [25] William J Blake, Mads Kærn, Charles R Cantor, and James J Collins. Noise in eukaryotic gene expression. Nature, 422:633–637, 2003.
- [26] Jonathan M Raser and Erin K O’Shea. Noise in gene expression: origins, consequences, and control. Science, 309:2010–2013, 2005.
- [27] Arjun Raj and Alexander van Oudenaarden. Nature, nurture, or chance: stochastic gene expression and its consequences. Cell, 135:216–226, 2008.
- [28] Mads Kærn, Timothy C Elston, William J Blake, and James J Collins. Stochasticity in gene expression: from theories to phenotypes. Nature Reviews Genetics, 6:451–464, 2005.
- [29] Abhyudai Singh and Mohammad Soltani. Quantifying intrinsic and extrinsic variability in stochastic gene expression models. PLoS One, 8:e84301, 2013.
- [30] Johan Paulsson. Models of stochastic gene expression. Physics of Life Reviews, 2:157–175, 2005.
- [31] Nir Friedman, Long Cai, and X Sunney Xie. Linking stochastic dynamics to population distribution: an analytical framework of gene expression. Physical Review Letters, 97:168302, 2006.
- [32] Vahid Shahrezaei and Peter S Swain. Analytical distributions for stochastic gene expression. Proceedings of the National Academy of Sciences, 105:17256–17261, 2008.
- [33] Otto G Berg. A model for the statistical fluctuations of protein numbers in a microbial population. Journal of Theoretical Biology, 71:587–603, 1978.
- [34] Vlad Elgart, Tao Jia, Andrew T Fenley, and Rahul Kulkarni. Connecting protein and mRNA burst distributions for stochastic models of gene expression. Physical Biology, 8:046001, 2011.
- [35] David R. Rigney. Stochastic model of constitutive protein levels in growing and dividing bacterial cells. Journal of Theoretical Biology, 76:453 – 480, 1979.
- [36] Abhyudai Singh, Brandon Razooky, Chris D Cox, Michael L Simpson, and Leor S Weinberger. Transcriptional bursting from the HIV-1 promoter is a significant source of stochastic noise in HIV-1 gene expression. Biophysical Journal, 98:L32–L34, 2010.
- [37] Abhyudai Singh and Pavol Bokes. Consequences of mRNA transport on stochastic variability in protein levels. Biophysical Journal, 103:1087–1096, 2012.
- [38] Abhyudai Singh, Brandon S Razooky, Roy D Dar, and Leor S Weinberger. Dynamics of protein noise can distinguish between alternate sources of gene-expression variability. Molecular Systems Biology, 8:607, 2012.
- [39] Olivia Padovan-Merhar, Gautham P. Nair, Andrew G. Biaesch, Andreas Mayer, Steven Scarfone, Shawn W. Foley, Angela R. Wu, L. Stirling Churchman, Abhyudai Singh, and Arjun Raj. Single mammalian cells compensate for differences in cellular volume and DNA copy number through independent global transcriptional mechanisms. Molecular Cell, 58:339–352, 2015.
- [40] Khem Raj. Ghusinga and Abhyudai Singh. First-passage time calculations for a gene expression model. In IEEE 53rd Annual Conference on Decision and Control (CDC), pages 3047–3052, 2014.
- [41] Abhyudai Singh and John J Dennehy. Stochastic holin expression can account for lysis time variation in the bacteriophage . Journal of The Royal Society Interface, 11:20140140, 2014.
- [42] Laurence A. Baxter. Reliability applications of the relevation transform. Naval Research Logistics, 29:323–330, 1982.
- [43] Franco Pellerey, Moshe Shaked, and Joel Zinn. Nonhomogeneous poisson processes and logconcavity. Probability in the Engineering and Informational Sciences, 14:353–373, 2000.
- [44] Stephen Cooper and Charles E Helmstetter. Chromosome replication and the division cycle of Escherichia coli B/r. Journal of Molecular Biology, 31:519–540, 1968.
- [45] Stephen Cooper. Bacterial growth and division: biochemistry and regulation of prokaryotic and eukaryotic division cycles. Elsevier, 2012.
- [46] Sattar Taheri-Araghi. Self-consistent examination of Donachie’s constant initiation size at the single-cell level. Frontiers in Microbiology, 6:1349, 2015.
- [47] Andrea Giometto, Florian Altermatt, Francesco Carrara, Amos Maritan, and Andrea Rinaldo. Scaling body size fluctuations. Proceedings of the National Academy of Sciences, 110:4646–4650, 2013.
- [48] Andrew S Kennard, Matteo Osella, Avelino Javer, Sander Tans, Pietro Cicuta, and Marco Cosentino Lagomarsino. Individuality and universality in the growth-division laws of single E. coli cells. arXiv preprint arXiv:1411.4321, 2014.
- [49] Srividya Iyer-Biswas, Gavin E Crooks, Norbert F Scherer, and Aaron R Dinner. Universality in stochastic exponential growth. Physical Review Letters, 113:028101, 2014.
- [50] Erfei Bi and J Lutkenhaus. FtsZ regulates frequency of cell division in Escherichia coli. Journal of Bacteriology, 172:2765–2768, 1990.
- [51] Joe Lutkenhaus. Assembly dynamics of the bacterial MinCDE system and spatial regulation of the Z ring. Annual Reviews of Biochemisty, 76:539–562, 2007.
- [52] Harold P Erickson, David E Anderson, and Masaki Osawa. FtsZ in bacterial cytokinesis: cytoskeleton and force generator all in one. Microbiology and Molecular Biology Reviews, 74:504–528, 2010.
- [53] Piet AJ De Boer. Advances in understanding E. coli cell fission. Current Opinion in Microbiology, 13:730–737, 2010.
- [54] David W Adams and Jeff Errington. Bacterial cell division: assembly, maintenance and disassembly of the Z ring. Nature Reviews Microbiology, 7:642–653, 2009.
- [55] An-Chun Chien, Norbert S Hill, and Petra Anne Levin. Cell size control in bacteria. Current Biology, 22:R340–R349, 2012.
- [56] Tove Atlung, Anders Løbner-Olesen, and Flemming G Hansen. Overproduction of dnaa protein stimulates initiation of chromosome and minichromosome replication in Escherichia coli. Molecular and General Genetics MGG, 206:51–59, 1987.
- [57] Anders Løbner-Olesen, Kirsten Skarstad, Flemming G Hansen, Kaspar von Meyenburg, and Erik Boye. The DnaA protein determines the initiation mass of Escherichia coli K-12. Cell, 57:881–889, 1989.
- [58] Kazuhisa Sekimizu, David Bramhill, and Arthur Kornberg. ATP activates dnaA protein in initiating replication of plasmids bearing the origin of the E. coli chromosome. Cell, 50:259–265, 1987.
- [59] Ingvild Flåtten, Solveig Fossum-Raunehaug, Riikka Taipale, Silje Martinsen, and Kirsten Skarstad. The DnaA protein is not the limiting factor for initiation of replication in escherichia coli. PLoS Genetics, 11:e1005276, 2015.
- [60] Erik Boye, Trond Stokke, Nancy Kleckner, and Kirsten Skarstad. Coordinating DNA replication initiation with cell growth: differential roles for DnaA and SeqA proteins. Proceedings of the National Academy of Sciences, 93:12206–12211, 1996.
- [61] Norbert S. Hill, Ryosuke Kadoya, Dhruba K. Chattoraj, and Petra Anne Levin. Cell size and the initiation of DNA replication in bacteria. PLoS Genetics, 8:e1002549, 2012.
- [62] Amit Mukherjee, Chune Cao, and Joe Lutkenhaus. Inhibition of FtsZ polymerization by SulA, an inhibitor of septation in Escherichia coli. Proceedings of the National Academy of Sciences, 95:2885–2890, 1998.
- [63] Joshua W Modell, Alexander C Hopkins, and Michael T Laub. A DNA damage checkpoint in Caulobacter crescentus inhibits cell division through a direct interaction with FtsW. Genes & Development, 25:1328–1343, 2011.
- [64] Sonsoles Rueda, Miguel Vicente, and Jesús Mingorance. Concentration and assembly of the division ring proteins FtsZ, FtsA, and ZipA during the Escherichia coli cell cycle. Journal of Bacteriology, 185:3344–3351, 2003.
- [65] Chunlin Lu, Jesse Stricker, and Harold P Erickson. FtsZ from Escherichia coli, Azotobacter vinelandii, and Thermotoga maritima–quantitation, GTP hydrolysis, and assembly. Cell Motility and the Cytoskeleton, 40:71–86, 1998.
- [66] Yu Tanouchi, Anand Pai, Heungwon Park, Shuqiang Huang, Rumen Stamatov, Nicolas E. Buchler, and Lingchong You. A noisy linear map underlies oscillations in cell size and gene expression in bacteria. Nature, 523:357–360, 2015.
- [67] MHA Davis. Markov models and optimization, volume 49 of Monographs on Statistics and Applied Probability. Chapman & Hall, London, 1993.