Diversity-induced decoherence
Abstract
We analyze the effect of small-amplitude noise and heterogeneity in a network of coupled excitable oscillators with strong time scale separation. Using mean-field analysis, we uncover the mechanism of a new nontrivial effect — diversity-induced decoherence (DIDC) — in which heterogeneity modulates the mechanism of self-induced stochastic resonance to inhibit the coherence of oscillations. We argue that DIDC may offer one possible mechanism via which, in excitable neural systems, generic heterogeneity and background noise can synergistically prevent unwanted resonances that may be related to hyperkinetic movement disorders.
The role of disorder in the dynamics of complex networks has been extensively studied in terms of noise and diversity (i.e., heterogeneity) effects Jung and Mayer-Kress (1995); Liu and Wang (1999); Busch and Kaiser (2003); Gammaitoni et al. (1998); McDonnell and Abbott (2009); Pérez et al. (2010). For example, Shibata and Kaneko showed that heterogeneity enhances regularity in the collective dynamics of coupled map lattices, even if each element has chaotic dynamics Shibata and Kaneko (1997). Later on, Cartwright observed the emergence of collective network oscillations in a cubic lattice of locally coupled and diverse FitzHugh-Nagumo (FHN) units, none of which were individually in an oscillatory state Cartwright (2000). Tessone et al. demonstrated an amplification of the response of a coupled oscillator network to an external signal, driven by an optimal level of heterogeneity of its elements, and named this effect diversity-induced resonance (DIR) Tessone et al. (2006); Toral et al. (2009); Chen et al. (2009); Wu et al. (2010a, b); Patriarca et al. (2012); Tessone et al. (2013); Grace and Hütt (2014); Patriarca et al. (2015); Liang et al. (2020). Other authors showed that DIR can occur even in the absence of an external forcing Kamal and Sinha (2015); Scialla et al. (2021). Some of these studies concluded that stochastic resonance (SR) and DIR are substantially analogous phenomena Tessone et al. (2006, 2007), to the point that diversity may be viewed as a form of “quenched noise”.
Diversity in complex networks dynamics has been studied also in terms of its interaction with noise, by introducing in a system both types of disorder. Most of this research highlighted the possibility to amplify resonance effects caused by noise thanks to diversity optimization, and vice versa Boschi et al. (2001); Li et al. (2012); Li and Ding (2014); Gassel et al. (2007). Recently, Scialla et al. Scialla et al. (2022) showed that the impact of diversity on network dynamics can be significantly different from that of noise and may result in an antagonistic effect, depending on the specific network configuration. At the same time, however, various regions of synergy between the two types of disorder, giving rise to strong resonance effects, were observed. Also, it has been shown that diversity in a network of FHN neurons can enhance coherence resonance (CR) Zhou et al. (2001), which is a regular response (i.e., a limit cycle behavior) to an optimal noise amplitude Pikovsky and Kurths (1997), occurring when the system is bounded near the bifurcation thresholds Neiman et al. (1997); Liu et al. (2010).
Another form of noise-induced resonance is self-induced stochastic resonance (SISR), which has a different mechanism from CR for the emergence of regular oscillations DeVille et al. (2005); Yamakou and Jost (2019). SISR occurs when a small-amplitude noise perturbing the fast variable of an excitable system with a strong time scale separation results in the onset of coherent oscillations Muratov et al. (2005); Yamakou and Jost (2019). Due to the peculiarity of operating at relatively weak noise, SISR represents a particularly interesting case to study the effects of the interplay between noise and diversity. This is relevant to the potential role of SISR as a signal amplification mechanism in biological systems, given that diversity is inherent to networks of neurons or other cells.
In this Letter, we demonstrate that in contrast to previous literature, showing that network diversity can be optimized to enhance collective behaviors such as synchronization or coherence Shibata and Kaneko (1997); Cartwright (2000); Tessone et al. (2006); Toral et al. (2009); Chen et al. (2009); Wu et al. (2010a, b); Patriarca et al. (2012); Tessone et al. (2013); Grace and Hütt (2014); Patriarca et al. (2015); Liang et al. (2020); Kamal and Sinha (2015); Scialla et al. (2021); Tessone et al. (2007); Scialla et al. (2022); Zhou et al. (2001), the effect of diversity on SISR, instead, can only be antagonistic. This indicates that the enhancement or deterioration of a noise-induced resonance phenomenon by diversity strongly depends on the underlying mechanism.
We point out that not only constructive, but also destructive resonance effects may have significant biological consequences. For instance, an increasing number of studies on Parkinson’s disease Vogt Weisenhorn et al. (2016) indicate that dopaminergic neurons are characterized by a relatively high degree of heterogeneity, and disease progression is associated with the death of only one or a few specific dopaminergic neuron subpopulations, leading to a loss of neuron diversity with respect to healthy brain tissues. Thus, the role of diversity in biological systems might be also to inhibit unwanted resonances through compensatory mechanisms between different neuron sub-types, which can result in pathological conditions, if missing.
There is still a very limited understanding of the named phenomena from a complex systems modeling viewpoint, as previous works have focused mostly on systems and conditions that favor constructive resonance effects. In this work, we uncover diversity-induced decoherence (DIDC) mechanism, where, in contrast to its effect on CR, diversity deteriorates the coherence of oscillations due to SISR.
As a paradigmatic model with well-known biological relevance, we study the effects of diversity in a network of globally coupled FHN units Fitzhugh (1960); FitzHugh (1961); Nagumo et al. (1962):
| (4) |
Here represent the fast membrane potential and slow recovery current variables of the elements, respectively; the index stands for nodes; is the synaptic coupling strength; is the time scale separation between and and are constant parameters. Diversity is introduced by assigning to each network element a different value of , as specified below. The terms () are independent Gaussian noises with zero mean, standard deviation , and correlation function . The noise intensity applied to each neuron will be measured by .
The excitable regime where the network defined by Eqs. (4) has a unique and stable fixed point is the required deterministic state for the occurrence of SISR DeVille and Vanden-Eijnden (2007a); Yamakou and Jost (2018); Yamakou et al. (2020). When , the point becomes a fixed point of Eqs. (4), and is unique if and only if
| (5) |
For the fixed point to be stable, we must have and , where is the Jacobian matrix of the linearized Eqs. (1). Since , we have and only if
| (6) |
To ensure that the network defined by Eqs. (4) lies in the excitable regime required for SISR, in the following we set and . We also set , , and . To introduce diversity, the values of are drawn from a truncated Gaussian distribution in the interval , and are randomly assigned to network elements. The standard deviation and mean of the distribution measure diversity and how far the network is from the oscillatory regime (corresponding to ), respectively.
To study the effects of diversity on SISR analytically, we apply the mean field approach introducing the global variables and . Adapting the method used in Refs. Desai and Zwanzig (1978); Tessone et al. (2006); Scialla et al. (2022), we set in Eqs. (4), alongside the assumptions that , .
We further assume that the standard deviation of the distribution is small, allowing the approximation
| (7) |
where denotes an average over the neurons. We note that the Gaussian distribution of in the range is always truncated whenever a given value of and/or pushes out of bounds, especially when is very close to the boundaries of .
Using these assumptions and averaging Eqs. (4) over the neurons, we obtain the following dynamical equations for the global variables and :
| (11) |
where and . can be considered as a diversity parameter, in that it increases with diversity in the network and for a homogeneous system (). Noise effects are represented by a global white noise term with zero mean and correlation function .
When there is no noise in first equation of Eqs. (11), , then in the adiabatic limit , for any initial condition of Eqs. (11) the system relaxes to and then to , where and are the right and left stable branches of the -nullcline, respectively. Solving for , we get three real and ordered solutions, namely, , which are all functions of .
Inserting and in the equation for in Eqs. (11) gives
| (14) |
The first (second) equation of Eqs. (14) together with equation () governs the slow motion of down (up) the left (right) stable branch of the -nullcline (see Fig. 1) to the leading order arising on the time scale when .
Now, if we switch on the noise, i.e., with a small amplitude, , the first equation of Eqs. (14) is not valid all the way down to the stable fixed point (in fact, for SISR to occur, the point should never be reached, otherwise, the trajectory would be trapped in the basin of attraction of the stable fixed point for a long time, thereby invoking a Poissonian spike train, leading to the non-occurrence of SISR) which is located on the left stable branch of the -nullcline, i.e., (see Fig. 1). But the first equation of Eqs. (14) still governs the slow motion of until the well-defined point where a horizontal escape (invoked by noise) of a trajectory from the left stable branch of the -nullcline occurs.
The same dynamics occur for the second equation of Eqs.(14) except that horizontal escape from the right stable branch of the -nullcline certainly occurs with or without noise. This is because the right (unlike the left) stable branch of the -nullcline has no fixed point to trap the trajectories and destroy the regularity of spikes. Thus, our analysis focuses only on the stochastic dynamics of the trajectories on the left stable branch.
To understand the escape mechanism of a trajectory from the left stable branch of the -nullcline at the point , we consider the limit , where the time scale separation between and becomes very large and Eqs. (11) reduce to the 1D Langevin equation
| (15) |
In this limit, which comes from the solution of the first equation of Eqs.(14) is practically frozen and can be considered as a fixed parameter, its time variation providing only a contribution to the dynamics governed by Eq. (15). The function in Eq. (15) is an effective double-well potential parametrically dependent on :
| (16) | |||||
Based on large deviations theory Freidlin (2001a, b) and Kramers’ law Kramers (1940), we write down for Eqs. (11) the generic conditions for the occurrence of SISR in slow-fast dynamical systems in the standard form Yamakou (2018); Kuehn (2015) as follows Muratov et al. (2005); DeVille and Vanden-Eijnden (2007b); Yamakou and Jost (2018)
| (21) |
Here, () and () are, respectively, the minimum and maximum points of the -nullcline, (,) is the unique (and stable) fixed point of Eqs. (11), and is the value of that satisfies the first equation of Eqs.(14) and at which the trajectories escape almost surely from the left stable branch of the -nullcine. The left () and right () energy barriers of are
| (24) |
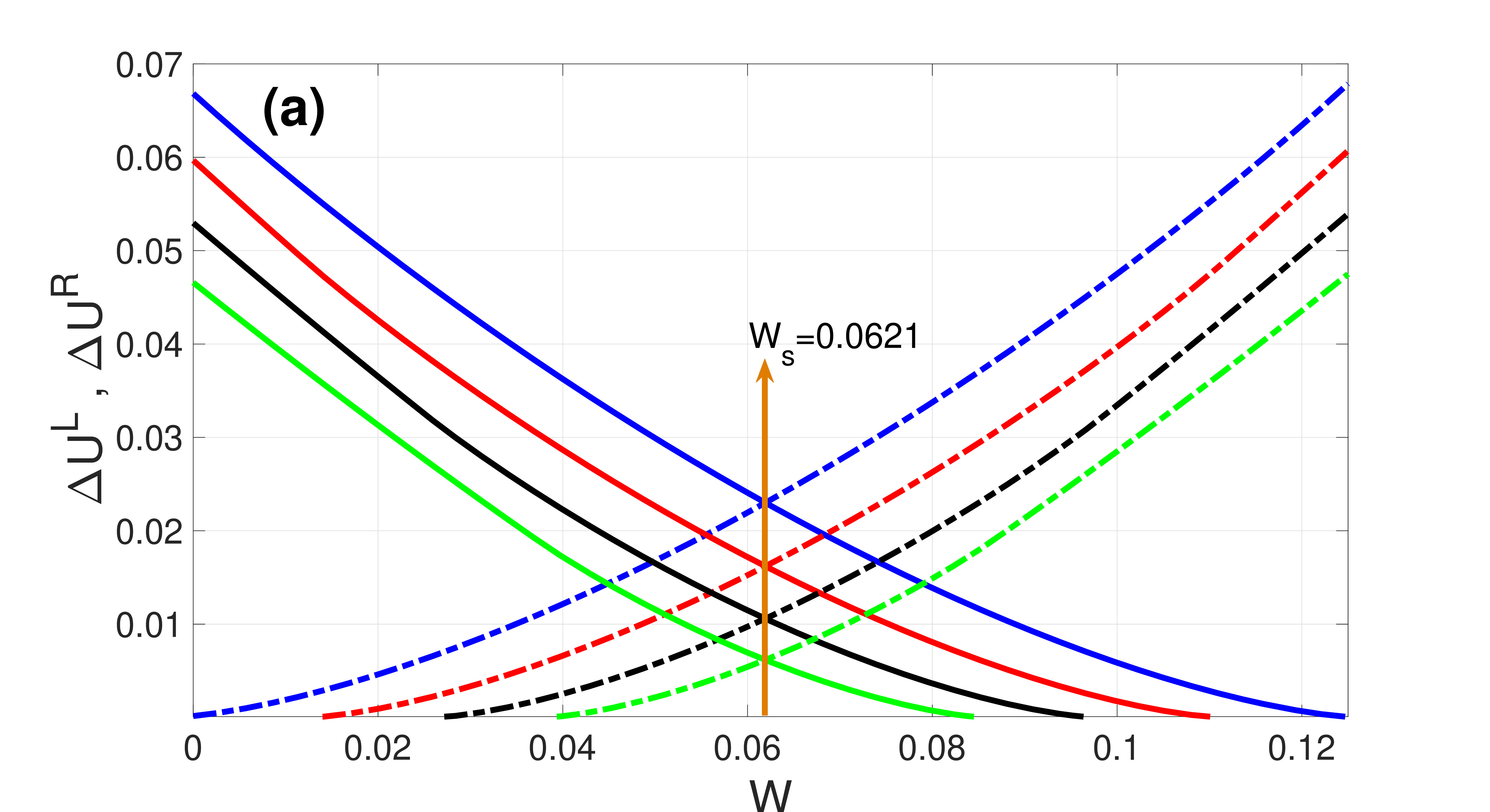
which are both non-negative and monotonic functions of , see Fig. 3(a).
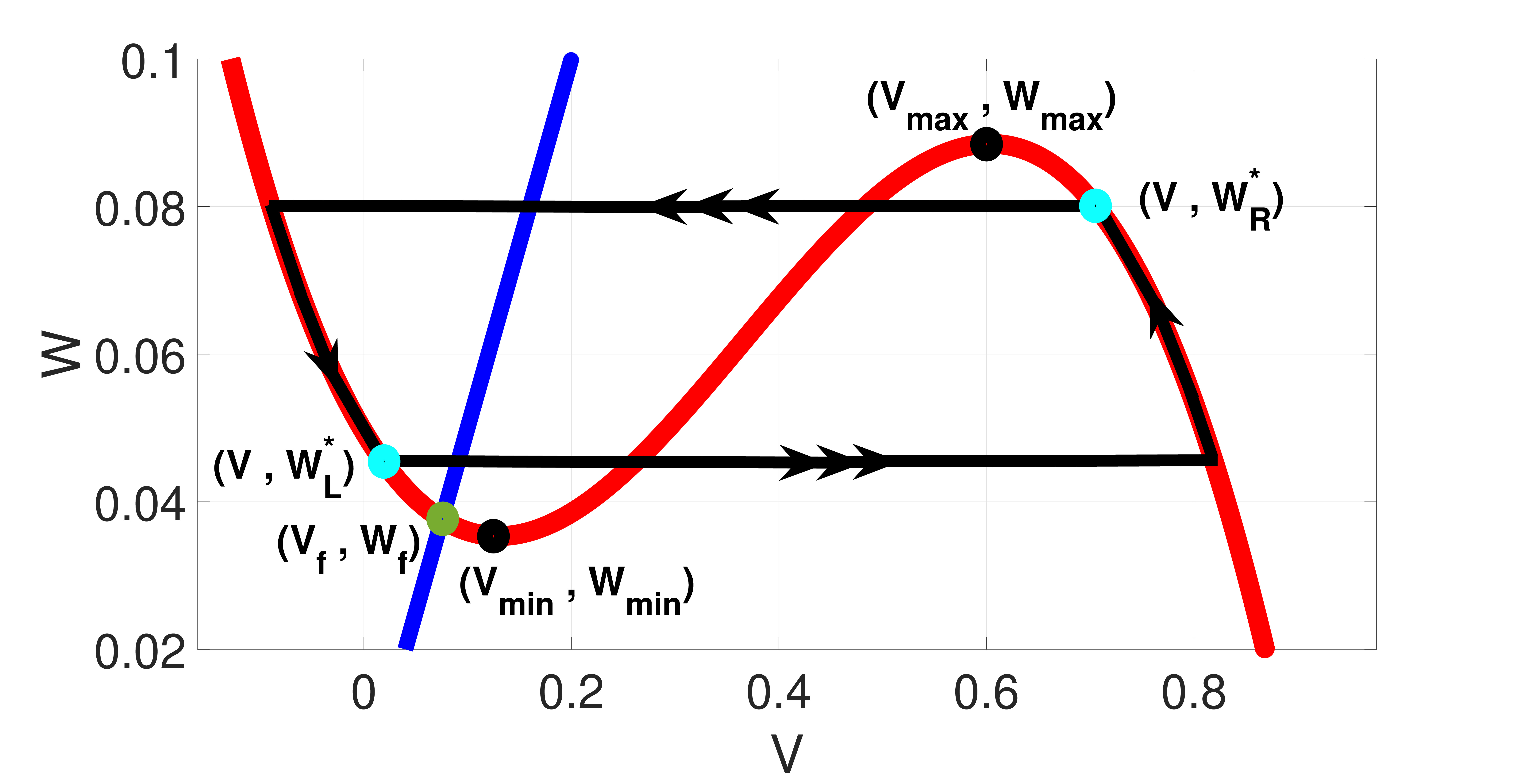
Figure 2 shows the landscape of and how varies with . We note that the asymmetry of is governed by and the double-well tends to disappear upon increasing , resulting in a loss of the bistability required for SISR occurrence. And represents the intersection point of and at , a point at which the two energy barriers are equal to each other. This happens when is symmetric at , i.e.,
| (25) |
At the point , the escape of a trajectory from the left stable branch and from the right stable branch of the -nullcline are both equally less probable.
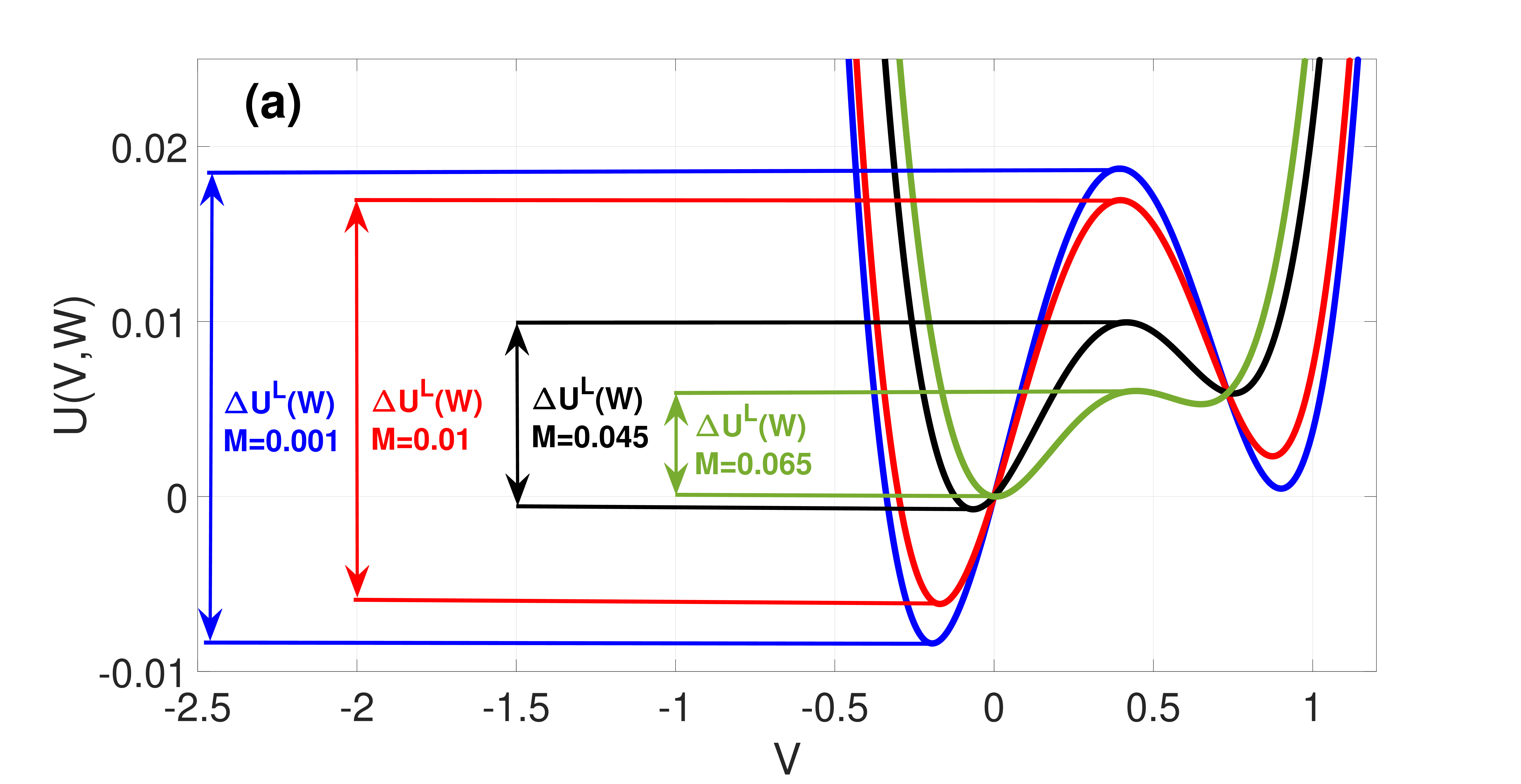
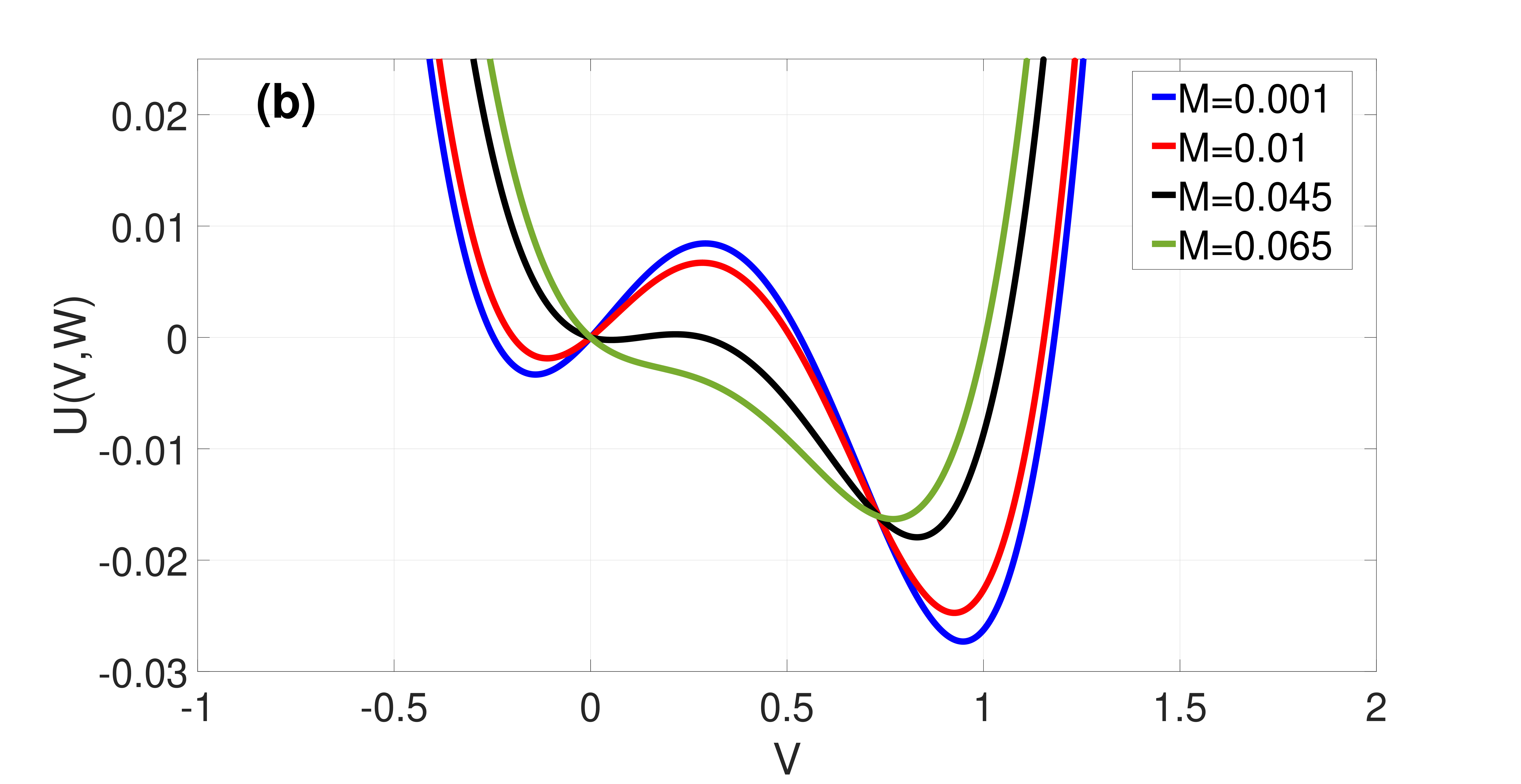
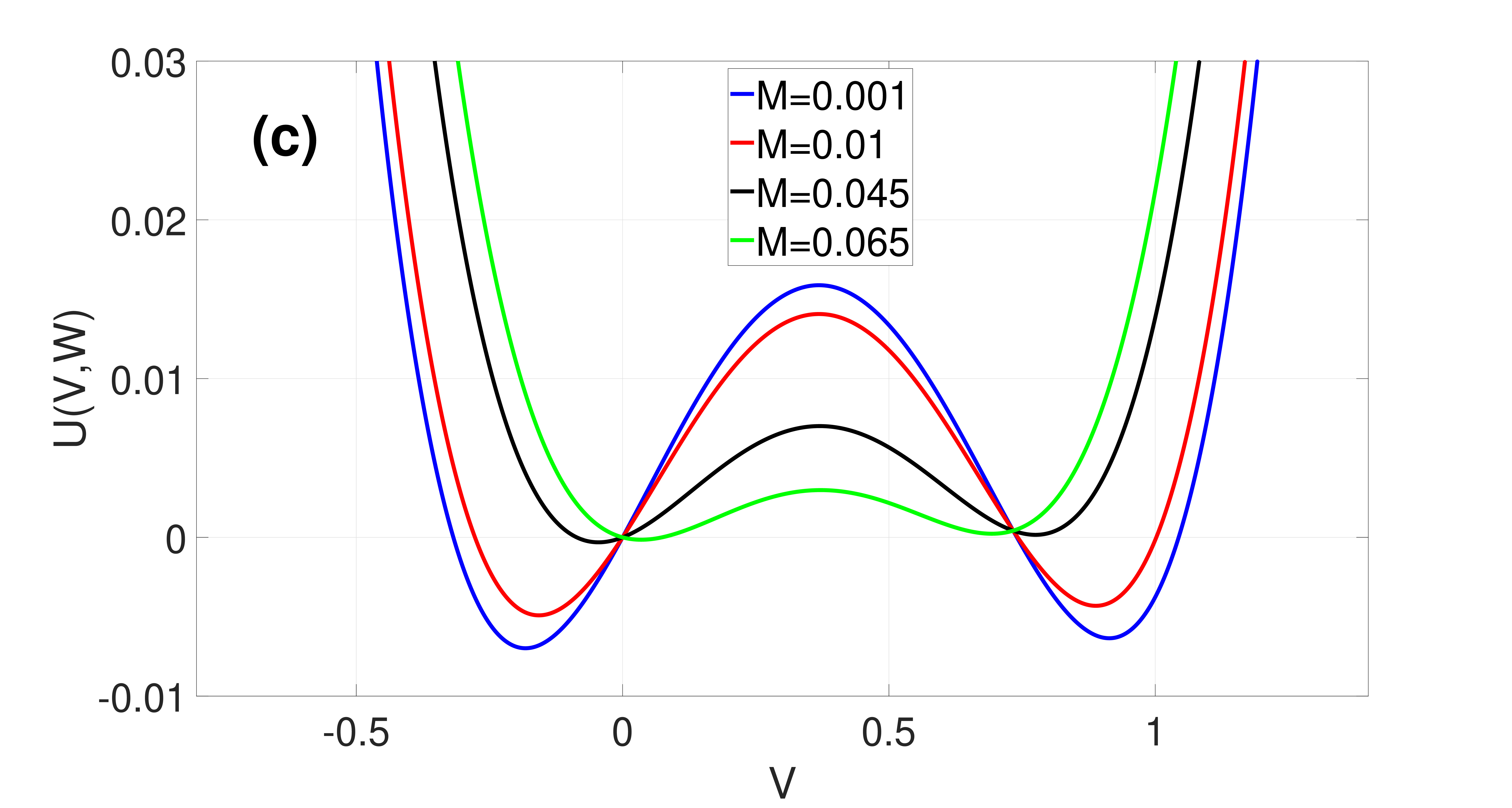
In (21), the first condition ensures that the fixed point is unique and stable; the second condition ensures that a trajectory can escape (almost surely) from the left stable branch of the -nullcline at the escape point ; the third condition ensures that the trajectory escapes before it reaches the stable fixed point, so that it does not get trapped into the basin of attraction of this fixed point for too long; and in the fourth condition, the monotonicity of and in the interval ensures that the escape points and on the left and right stable branches of the -nullcline are unique, which would in turn ensure the periodicity of the trajectory leading to coherent spiking.
Since is the lowest attainable point of a trajectory on the left stable branch of the -nullcline and the interval in the second condition in (21) is open, SISR deteriorates (i.e., the spiking becomes less coherent) and eventually disappears moving away from the center of the interval. Thus, for a given , we use the boundaries of this interval to calculate the minimum () and maximum () noise intensity between which the highest degree of SISR can be achieved:
| (26) |
The quantities and have a dependence on the diversity parameter through and . Thus, the length of the interval , can be controlled by . It is worth noting that when , diversity alone cannot induce SISR. This is because, no single neuron in the network can spike as long as the excitability parameter (which is also the heterogeneity parameter) lies in , i.e., the excitable regime.
The occurrence of SISR depends on whether the parameter values of the system, including , satisfy the four conditions (21) in the double limit . Hence, it suffices to study the variation of versus to uncover the effect of diversity on the degree of SISR. This is done in Fig. 3, showing that decreases upon increasing . Thus, DIDC occurs when diversity in the network increases, leading to a deterioration and eventually destruction of the coherence of the spike train due to SISR, by shrinking the length of the interval , toward zero.
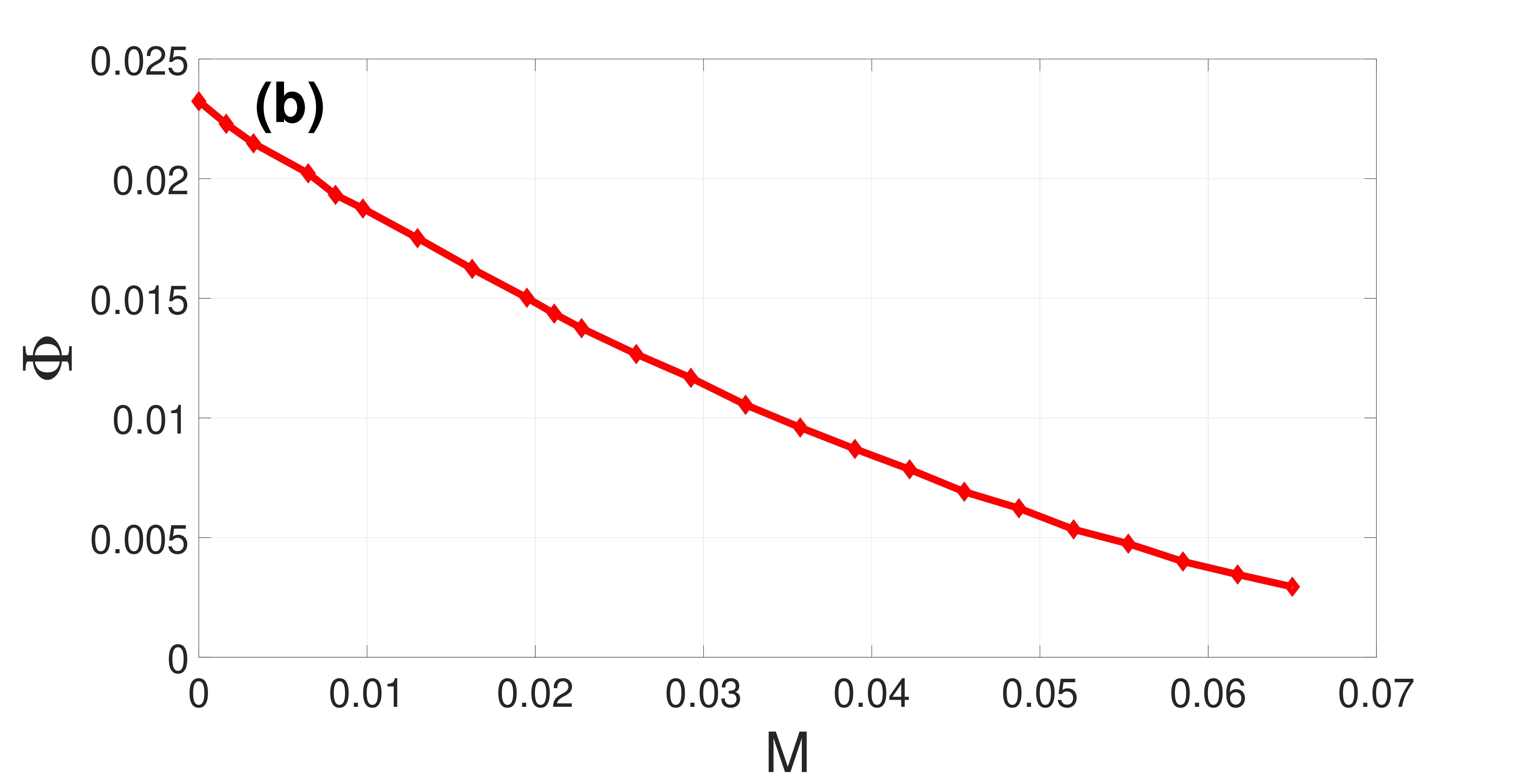
We corroborate the theoretical analysis via numerical simulations. We numerically integrate Eqs. (4) for neurons using the fourth-order Runge-Kutta algorithm for stochastic processes Kasdin (1995) and the Box-Muller algorithm Knuth (1973). The integration time step is and the total simulation time is . For each realization, we choose for the th neuron random initial conditions , with uniform probability in the ranges and . After an initial transient time , we start recording the neuron spiking times ( counts the spiking times). Averages are taken over 15 realizations, which warrant appropriate statistical accuracy.
We illustrate the effect of diversity, synaptic noise, and distance of the excitable network from the oscillatory regime, measured by , , and , respectively, on the degree of coherence of the spikes induced by SISR. We use the coefficient of variation () given by the normalized standard deviation of the mean interspike interval (ISI) Pikovsky and Kurths (1997). For coupled neurons, is given by Masoliver et al. (2017)
| (27) |
where and , with and representing the mean and mean squared ISI (over time), , of neuron .
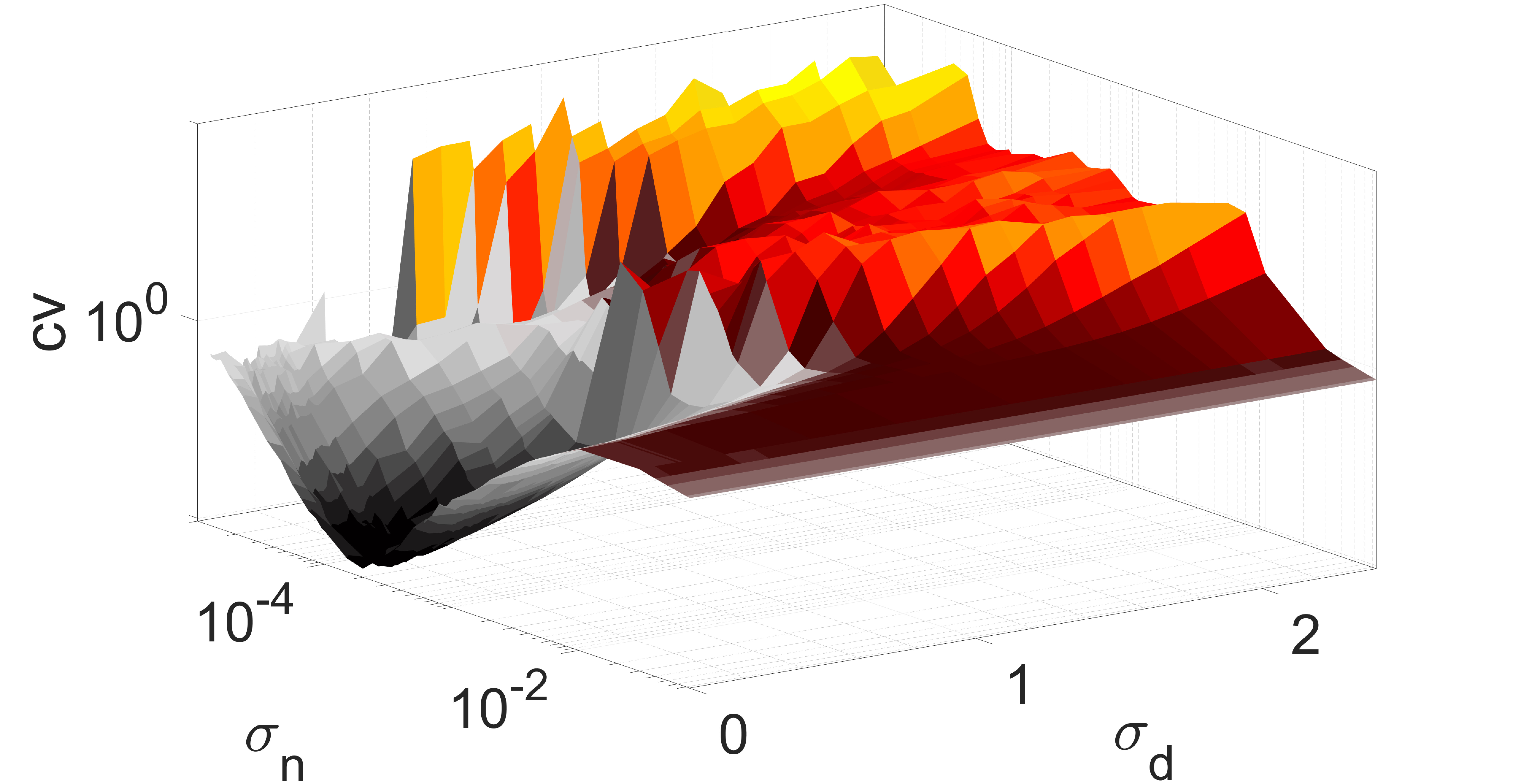
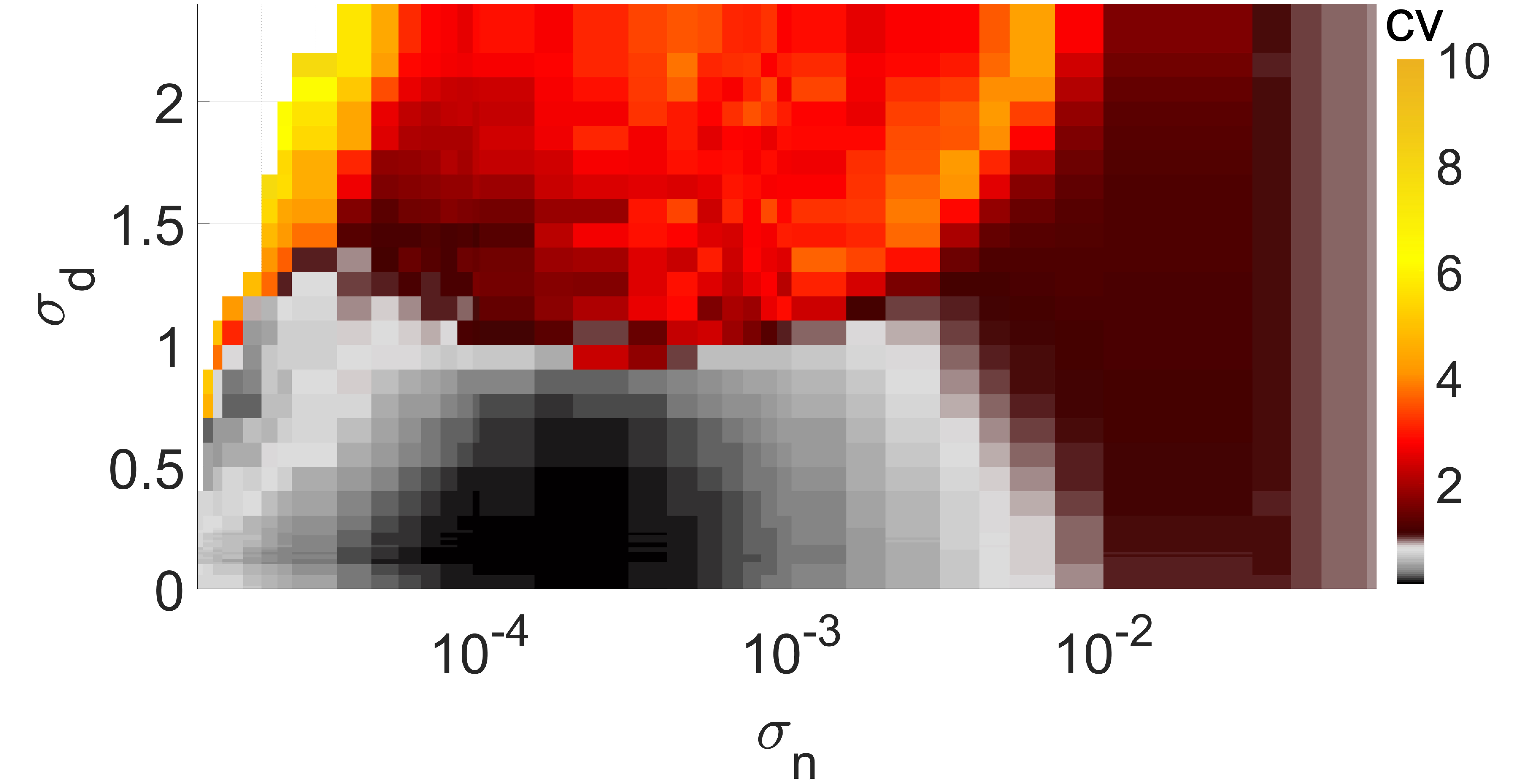
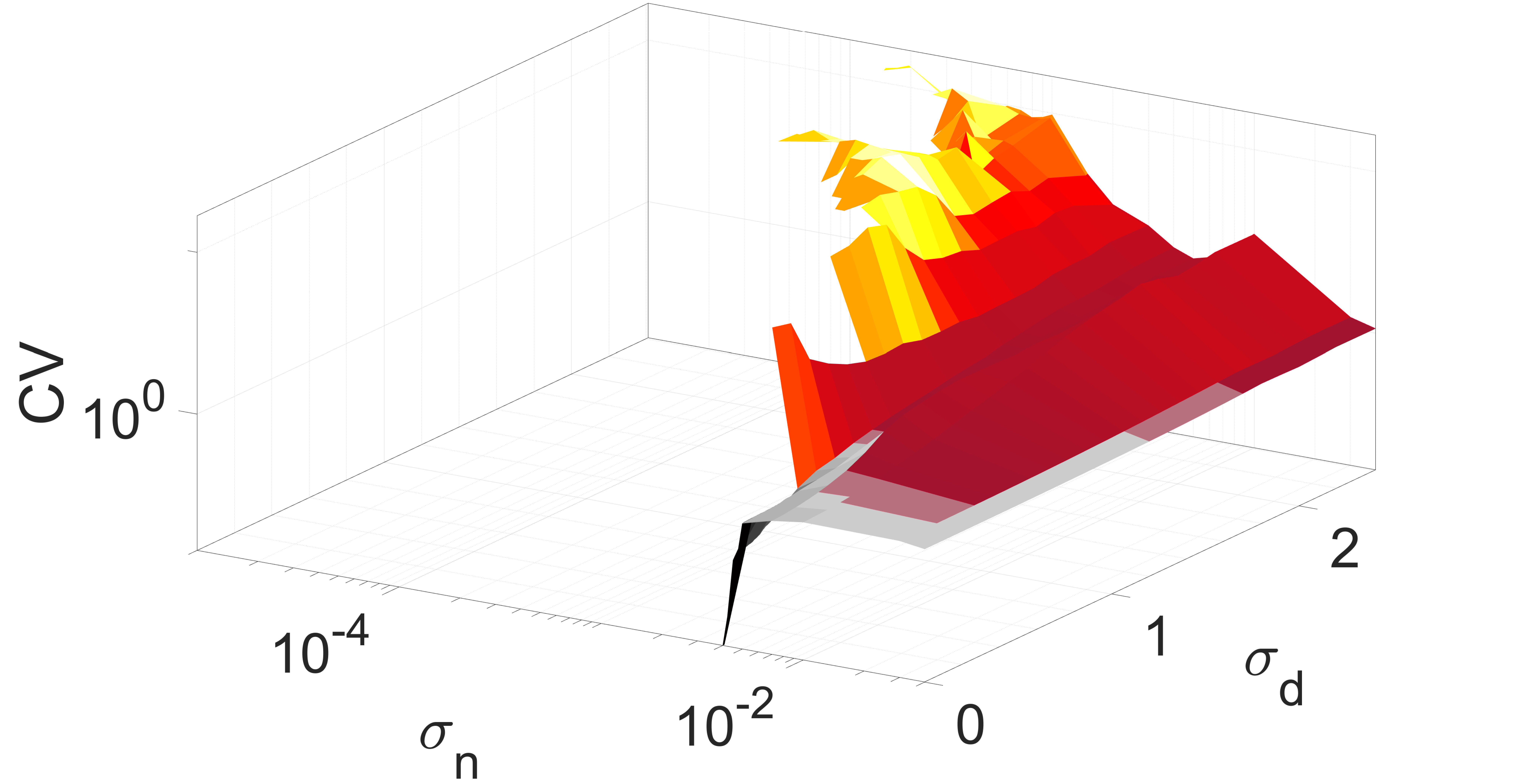
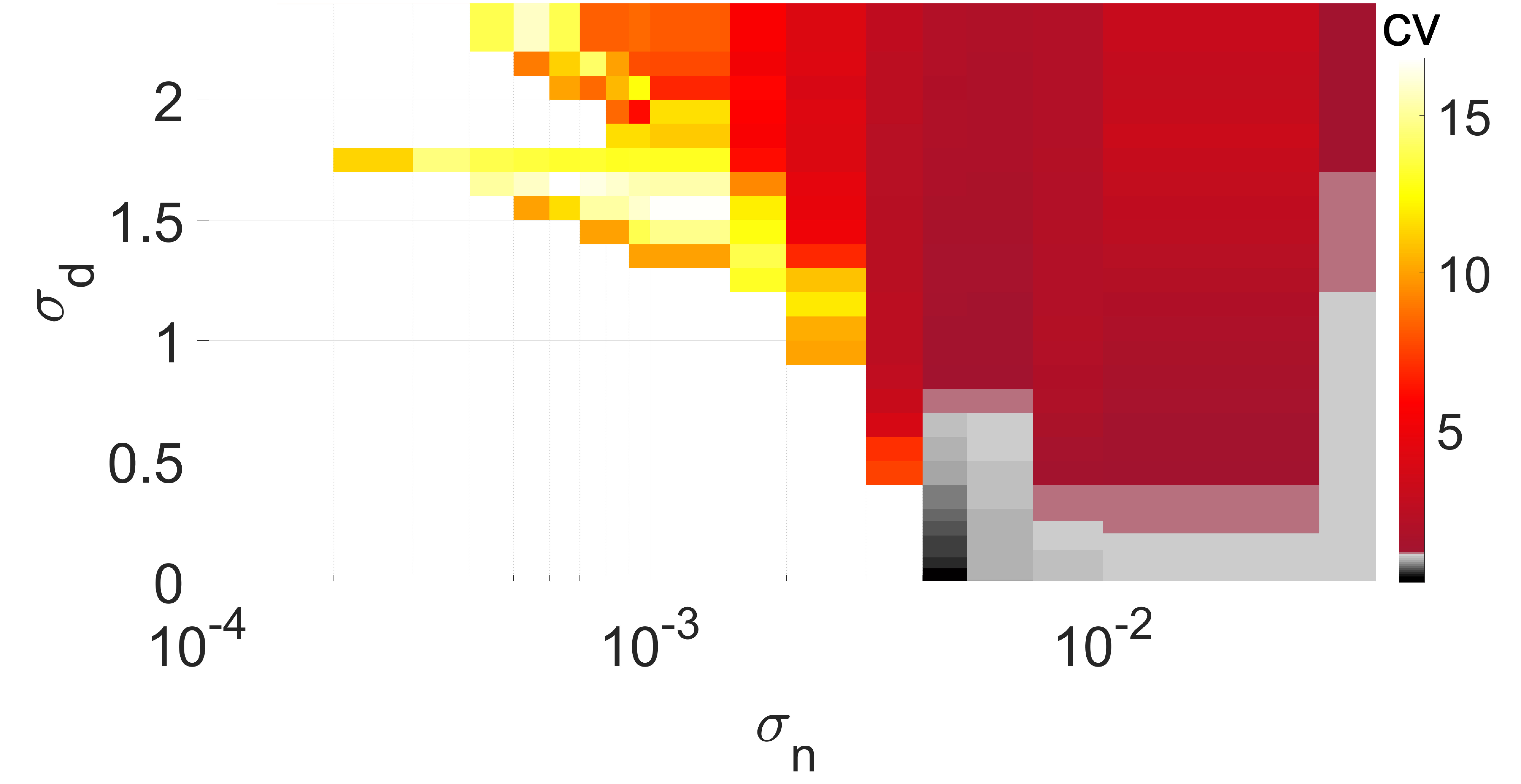
We determine the spike occurrence times from the instant the membrane potential variable crosses the threshold . The will be the higher the more variable the mean ISIs are. Thus, since Poisson spike train events are independent and all have a normalized standard deviation of unity (i.e., ), they can be used as reference for the average variability of spike trains of the network Gabbiani and Koch (1998). When , the average variability of spike trains of the network is more variable than a Poisson process. When , the average spiking activity of the network becomes more coherent, with corresponding to perfectly periodic spike trains. The degree of coherence is illustrated in Fig. 4, which depicts against the synaptic noise and diversity parameter at two different values of .
In Fig. 4(a), the mean value is close to the lower bound of the interval , i.e., close to the oscillatory regime. It can be observed that when and , we have a low , indicating a high degree of coherence due to SISR. For , the interval in which has shrunk to zero, i.e., for all values, indicating a significant deterioration and eventual destruction of the coherence as increases.
In Fig. 4(b), the mean of the diversity distribution is fixed at a higher value . In this case, the unique fixed point becomes even more stable than in Fig. 4(a). Small diversities and weak synaptic noise intensities are not strong enough to induce spiking; thus the network remains inactive and the value of is undefined.
For and , neurons respond differently to the synaptic noise due to the diverse strengths of the excitable regimes. Due to the all-to-all coupling in the network, the large diversity boosts the weak synaptic noise, leading to the production of spikes. However, because the diversity is large, the conditions required for SISR are violated and the spikes produced are incoherent — see in Fig. 4(b) the magenta region bounded by and , where . At a relatively stronger synaptic noise intensity, i.e., and a very small diversity of , the degree of coherence due to SISR is best and . As increases while the synaptic noise is fixed at , the degree of SISR deteriorates and .
The results in Fig. 4 were obtained for a specific value of the time scale parameter (), which is a crucial parameter for SISR. Moreover, additional simulations performed for other values of and (not shown) lead to qualitatively similar results.
(a)
(b)
In conclusion, we have provided evidence that there are complex network configurations and parameter regimes where diversity can only cause a deterioration of well-known resonance phenomena, such as SISR. This is predicted by our mean field analysis and confirmed by numerical simulations.
The decoherence effect appears as soon as there is a minimal degree of diversity in the system and rapidly grows up to a complete resonance muting as diversity increases. The basic mechanism of this effect is that diversity causes a partial or complete disappearance of the energy barrier in the mean field double-well potential, responsible for the coherent spiking corresponding to SISR. The fact that in this system diversity cannot be optimized to enhance coherence, but can only disrupt it, is a nontrivial result. This is because the possibility to adjust diversity in order to amplify collective network behaviors has been previously demonstrated across a broad range of network types, configurations and conditions and is, therefore, a very general phenomenon Shibata and Kaneko (1997); Cartwright (2000); Tessone et al. (2006); Toral et al. (2009); Chen et al. (2009); Wu et al. (2010a, b); Patriarca et al. (2012); Tessone et al. (2013); Grace and Hütt (2014); Patriarca et al. (2015); Liang et al. (2020); Kamal and Sinha (2015); Scialla et al. (2021); Tessone et al. (2007); Scialla et al. (2022).
We have illustrated the effect of DIDC in a prototypical excitable model network, which suggests that the effect may be common to other physical, chemical, and biological systems. Based on our analysis and on experimental evidence that a neuron diversity loss can be associated to hyperkinetic disorders characterized by involuntary movements, we hypothesize that diversity may be used in biological systems not only to amplify weak signals, as suggested by previous literature, but also as an efficient control mechanism to prevent undesired resonances.
M.E.Y acknowledges support from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project No. 456989199. E.H., M.P, and S.S. acknowledge support from the Estonian Research Council through Grant PRG1059.
References
- Jung and Mayer-Kress (1995) P. Jung and G. Mayer-Kress, Physical Review Letters 74, 2130 (1995).
- Liu and Wang (1999) F. Liu and W. Wang, Journal of the Physical Society of Japan 68, 3456 (1999).
- Busch and Kaiser (2003) H. Busch and F. Kaiser, Physical Review E 67, 041105 (2003).
- Gammaitoni et al. (1998) L. Gammaitoni, P. Hänggi, P. Jung, and F. Marchesoni, Reviews of Modern Physics 70, 223 (1998).
- McDonnell and Abbott (2009) M. D. McDonnell and D. Abbott, PLoS Computational Biology 5, e1000348 (2009).
- Pérez et al. (2010) T. Pérez, C. R. Mirasso, R. Toral, and J. D. Gunton, Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 368, 5619 (2010).
- Shibata and Kaneko (1997) T. Shibata and K. Kaneko, EPL (Europhysics Letters) 38, 417 (1997).
- Cartwright (2000) J. H. Cartwright, Physical Review E 62, 1149 (2000).
- Tessone et al. (2006) C. J. Tessone, C. R. Mirasso, R. Toral, and J. D. Gunton, Physical Review Letters 97, 194101 (2006).
- Toral et al. (2009) R. Toral, E. Hernandez-Garcia, and J. D. Gunton, International Journal of Bifurcation and Chaos 19, 3499 (2009).
- Chen et al. (2009) H. Chen, Z. Hou, and H. Xin, Physica A: Statistical Mechanics and its Applications 388, 2299 (2009).
- Wu et al. (2010a) L. Wu, S. Zhu, and X. Luo, Chaos: An Interdisciplinary Journal of Nonlinear Science 20, 033113 (2010a).
- Wu et al. (2010b) L. Wu, S. Zhu, X. Luo, and D. Wu, Physical Review E 81, 061118 (2010b).
- Patriarca et al. (2012) M. Patriarca, S. Postnova, H. A. Braun, E. Hernández-García, and R. Toral, PLoS Computational Biology 8, e1002650 (2012).
- Tessone et al. (2013) C. J. Tessone, A. Sánchez, and F. Schweitzer, Physical Review E 87, 022803 (2013).
- Grace and Hütt (2014) M. Grace and M.-T. Hütt, The European Physical Journal B 87, 1 (2014).
- Patriarca et al. (2015) M. Patriarca, E. Hernández-García, and R. Toral, Chaos, Solitons & Fractals 81, 567 (2015).
- Liang et al. (2020) X. Liang, X. Zhang, and L. Zhao, Chaos: An Interdisciplinary Journal of Nonlinear Science 30, 103101 (2020).
- Kamal and Sinha (2015) N. K. Kamal and S. Sinha, Pramana 84, 249 (2015).
- Scialla et al. (2021) S. Scialla, A. Loppini, M. Patriarca, and E. Heinsalu, Physical Review E 103, 052211 (2021).
- Tessone et al. (2007) C. J. Tessone, A. Scire, R. Toral, and P. Colet, Physical Review E 75, 016203 (2007).
- Boschi et al. (2001) C. D. E. Boschi, E. Louis, and G. Ortega, Physical Review E 65, 012901 (2001).
- Li et al. (2012) Y.-Y. Li, B. Jia, H.-G. Gu, and S.-C. An, Communications in Theoretical Physics 57, 817 (2012).
- Li and Ding (2014) Y.-Y. Li and X.-L. Ding, Communications in Theoretical Physics 62, 917 (2014).
- Gassel et al. (2007) M. Gassel, E. Glatt, and F. Kaiser, Physical Review E 76, 016203 (2007).
- Scialla et al. (2022) S. Scialla, M. Patriarca, and E. Heinsalu, Europhysics Letters 137, 51001 (2022).
- Zhou et al. (2001) C. Zhou, J. Kurths, and B. Hu, Physical Review Letters 87, 098101 (2001).
- Pikovsky and Kurths (1997) A. S. Pikovsky and J. Kurths, Physical Review Letters 78, 775 (1997).
- Neiman et al. (1997) A. Neiman, P. I. Saparin, and L. Stone, Physical Review E 56, 270 (1997).
- Liu et al. (2010) Z.-Q. Liu, H.-M. Zhang, Y.-Y. Li, C.-C. Hua, H.-G. Gu, and W. Ren, Physica A: Statistical Mechanics and its Applications 389, 2642 (2010).
- DeVille et al. (2005) R. L. DeVille, E. Vanden-Eijnden, and C. B. Muratov, Physical Review E 72, 031105 (2005).
- Yamakou and Jost (2019) M. E. Yamakou and J. Jost, Physical Review E 100, 022313 (2019).
- Muratov et al. (2005) C. B. Muratov, E. Vanden-Eijnden, and E. Weinan, Physica D: Nonlinear Phenomena 210, 227 (2005).
- Vogt Weisenhorn et al. (2016) D. M. Vogt Weisenhorn, F. Giesert, and W. Wurst, Journal of neurochemistry 139, 8 (2016).
- Fitzhugh (1960) R. Fitzhugh, The Journal of general physiology 43, 867 (1960).
- FitzHugh (1961) R. FitzHugh, Biophysical Journal 1, 445 (1961).
- Nagumo et al. (1962) J. Nagumo, S. Arimoto, and S. Yoshizawa, Proceedings of the IRE 50, 2061 (1962).
- DeVille and Vanden-Eijnden (2007a) R. L. DeVille and E. Vanden-Eijnden, Communications in Mathematical Sciences 5, 431 (2007a).
- Yamakou and Jost (2018) M. E. Yamakou and J. Jost, Nonlinear Dynamics 93, 2121 (2018).
- Yamakou et al. (2020) M. E. Yamakou, P. G. Hjorth, and E. A. Martens, Frontiers in Computational Neuroscience 14, 62 (2020).
- Desai and Zwanzig (1978) R. C. Desai and R. Zwanzig, Journal of Statistical Physics 19, 1 (1978).
- Freidlin (2001a) M. I. Freidlin, Journal of Statistical Physics 103, 283 (2001a).
- Freidlin (2001b) M. Freidlin, Stochastics and Dynamics 1, 261 (2001b).
- Kramers (1940) H. A. Kramers, Physica 7, 284 (1940).
- Yamakou (2018) M. E. Yamakou, Weak-noise-induced phenomena in a slow-fast dynamical system, Ph.D. thesis, Max Planck Institute for Mathematics in the Sciences, Max Planck Society (2018).
- Kuehn (2015) C. Kuehn, Multiple time scale dynamics, Vol. 191 (Springer, 2015).
- DeVille and Vanden-Eijnden (2007b) R. DeVille and E. Vanden-Eijnden, Journal of Statistical Physics 126, 75 (2007b).
- Kasdin (1995) N. J. Kasdin, Journal of Guidance, Control, and Dynamics 18, 114 (1995).
- Knuth (1973) D. E. Knuth, Reading, MA , 51 (1973).
- Masoliver et al. (2017) M. Masoliver, N. Malik, E. Schöll, and A. Zakharova, Chaos: An Interdisciplinary Journal of Nonlinear Science 27, 101102 (2017).
- Gabbiani and Koch (1998) F. Gabbiani and C. Koch, Methods in Neuronal Modeling 12, 313 (1998).