Evidence for charge and spin order in single crystals of La3Ni2O7 and La3Ni2O6
Abstract
Charge and spin order is intimately related to superconductivity in copper oxide superconductors. To elucidate the competing orders in various nickel oxide compounds are crucial given the fact that superconductivity has been discovered in Nd0.8Sr0.2NiO2 films. Herein, we report structural, electronic transport, magnetic, and thermodynamic characterizations on single crystals of La3Ni2O7 and La3Ni2O6. La3Ni2O7 is metallic with mixed Ni2+ and Ni3+ valent states. Resistivity measurements yield two transition-like kinks at 110 and 153 K, respectively. The kink at 153 K is further revealed from magnetization and specific heat measurements, indicative of the formation of charge and spin order. La3Ni2O6 single crystals obtained from topochemical reduction of La3Ni2O7 are insulating and show an anomaly at 176 K on magnetic susceptibility. The transition-like behaviors of La3Ni2O7 and La3Ni2O6 are analogous to the charge and spin order observed in La4Ni3O10 and La4Ni3O8, suggesting charge and spin order is a common feature in the ternary La-Ni-O system with mixed-valent states of nickel.
I Introduction
The recent discovery of superconductivity in Nd0.8Sr0.2NiO2 films has aroused great research enthusiasm in the search for superconductivity and understanding the pairing mechanism in nickel oxide systemLi et al. (2019). Particularly, whether the mechanisms of the superconductivity in nickelates and copper oxide superconductors are the same remaining an open question. The superconductivity, spin order, and charge order could be tuned by carrier doping in cuprates. It is widely accepted that the static and dynamic spin and charge orders play a crucial role in the mechanism of superconductivityVignolle et al. (2007); Keimer et al. (2015).
The ternary nickel oxide -Ni-O system ( = La, Pr, Nd, Sm, and Eu) contains the Ruddlesden-Popper (RP) compounds NinO3n+1, which possess layers of perovskite-type NiO3, separated by single rocksalt O layer along the axisZhang et al. (2020a, b); Wang et al. (2020); Lee and Pickett (2004). The Ni ions exhibit mixed valences of Ni3+ (3) and Ni2+ (3). By a topochemical reduction method, two apical oxygen atoms of a NiO6 octahedra could be removed and the remained oxygen atoms rearrange and result in a fluorite -O2- layer, as shown in Fig. 1. The topochemical reduced compounds NinO2n+2 with mixed-valent states of Ni1+ (3) and Ni2+ would form. The structures of the RP system and the PR reduced system are analogous to the ternary -Cu-O system, especially the Ni-O planes that are regarded as alternative superconducting planes like the Cu-O planes in cuprates. Theorists suggested that superconductivity may be induced by doping low-spin Ni2+ () to a nickelate antiferromagnetic (AF) insulator with Ni1+ () in a square planar coordination with O ionsAnisimov et al. (1999). This could be realized in the chemical reduced RP phase NinO2n+2 by hole doping on the site, such as Sr doped Sr0.2NiO2, where =La, Nd, and Sm. Superconductivity has been indeed observed in films of these hole doped compounds and Nd6Ni5O12, where nickel ions exhibit an average valence of +1.2Gu and Wen (2022); Pan et al. (2022). The transition temperature of the superconducting films could be enhanced by pressureWang et al. (2021). However, superconductivity has not been observed in bulk samples of the -Ni-O system under ambient or high pressureWang et al. (2020); He et al. (2021); Li et al. (2020a); Huo et al. (2022).
| n | NinO3n+1 | NinO2n+2 | ||
|---|---|---|---|---|
| composition | Ni valence | composition | Ni valence | |
| 113 | +3 | 112 | +1 | |
| 3 | 4310 | +2.67 | 438 | +1.33 |
| 2 | 327 | +2.5 | 326 | +1.5 |
| 1 | 214 | +2 | - | |
Progress on studies of the charge and spin order has been made due to the availability of high-quality single crystals for the La-Ni-O system grown by the floating zone technique with high oxygen pressure. AF transitions were identified on metallic LaNiO3 (n=) and La4Ni3O10 (n=3) single crystals by neutron scattering studies, which were absent for previous measurements on powder samplesGuo et al. (2018); Zhang et al. (2020c); Garc´ıa-Muñoz et al. (1992). The AF transition has been ascribed to spin density wave (SDW) that originates from Fermi surface nesting, differing from the spin order in doped La2-xSrxNiO4 and cuprates. A charge density wave (CDW) coincident with the SDW was found in La4Ni3O10 and was suggested to result in a metal-to-metal transition. For the topochemically reduced product La4Ni3O8, synchrotron X-ray and neutron diffraction also reveal stacked charge and spin stripe weakly correlated along the axisZhang et al. (2016, 2019). In this case, La4Ni3O8 is an insulator and the charge and spin order results from the competition between the Coulomb repulsion, spin orbital coupling, and magnetic exchange interaction.
The n=2 RP compound La3Ni2O7 and chemical reduced product La3Ni2O6 consisting of the bilayer NiO2 planes are analogous to the trilayer La4Ni3O10 and La4Ni3O8 in structural and physical propertiesSreedhar et al. (1994); Zhang et al. (1994); Taniguchi et al. (1995); Kobayashi et al. (1996); Ling et al. (1999); Zhang and Greenblatt (1995). Theoretical calculations for La3Ni2O7 and La4Ni3O10 suggest existence of a hidden one-dimensional Fermi surface nesting which would result in CDW instabilityWu et al. (2001); Seo et al. (1996). For La3Ni2O6 and La4Ni3O8, charge-ordered related structural distortion and magnetic order will emerge in the ground stateBotana et al. (2016). The electronic density of states of La3Ni2O7 indeed has an abrupt change at around K, reflected in both the Hall coefficient and Seebeck coefficientSreedhar et al. (1994); Taniguchi et al. (1995); Kobayashi et al. (1996). However, neutron diffractionLing et al. (1999), resistivity, and magnetic susceptibility measurements on powder samples of La3Ni2O7 that were synthesized through the soft chemistry method did not reveal evidence of charge or spin orderSreedhar et al. (1994); Zhang et al. (1994); Taniguchi et al. (1995); Kobayashi et al. (1996); Ling et al. (1999). La3Ni2O6 has raised great interest because of the similarities of the electronic structures to cuprates and possible superconductivity through tuning the valence by carrier doping or high pressurePoltavets et al. (2009). Moreover, NMR study of La3Ni2O6 suggests the existence of magnetic interactionsCrocker et al. (2013), but the basic magnetic properties are still not clear. As a matter of fact, a comprehensive study of La3Ni2O7 and La3Ni2O6 is lacking due to the unavailability of single crystals. Single crystal growth of the samples with the average valences of nickel ions larger than +2 requires high pressure oxygen and the pressure window is narrow for a specific compoundZhang et al. (2020d). However, single crystals are crucial for investigations of the possible emerging orders of charge and spin in La3Ni2O7 and La3Ni2O6 in order to ascertain the universality for the ternary nickel oxide system, and pave a way for further manipulation of the states of nickel, even for realization of superconductivityPoltavets et al. (2009).
Here, we report successful growth of La3Ni2O7 single crystals by a high oxygen pressure floating zone technique and the achievement of La3Ni2O6 single crystals through a subsequent low temperature topochemical reduction methodZhang et al. (2020d). Electrical resistivity measurements on the single crystals of La3Ni2O7 and La3Ni2O6 show significantly different properties. La3Ni2O7 is metallic while La3Ni2O6 is insulating. Superconductivity has not been realized in La3Ni2O6 under pressure up to 25.3 GPa. Anomalies in resistivity, susceptibility, and specific heat that may correspond to spin and charge order have been observed in both compounds. The results indicate the emergent order of charge and spin is universal for the nickelates with mixed-valent states of nickel.
II Experimental Methods
Polycrystalline samples were synthesized through the standard solid-state reaction techniques. Stoichiometric amounts of La2O3 and an excess of 0.5% NiO (Macklin, 99.99%) were thoroughly ground. The excess of 0.5% NiO was used to compensate the loss of volatilization. The ground mixtures were made into pellets and sintered at 1100 ∘C for 50 h. After cooling down to room temperature, the reactants were reground and made into pellets for sintering again. The procedures were repeated for 3 times to ensure a complete and homogeneous reactionZhang et al. (2020d). The seed and feed rods were prepared by pressuring the powders under hydrostatic pressure and sintered at 1400 ∘C for 12 h. The rods were approximate 9 cm in length and 0.6 cm in diameter.
High oxygen pressure is crucial for synthesis of the homologous RP system of nickelates. A vertical optical-image floating-zone furnace designed for a 100 bar high pressure (HKZ, SciDre, Dresden) was employed in our single crystal growth. La3Ni2O7 single crystals were grown with an oxygen pressure at p(O2)=15 bar and a 5 kW Xenon arc lamp. The traveling rate was 3 mm/h after a fast traveling procedure at 10 mm/h to improve the density. After that, the feed and seed rods counter-rotate at 15 and 10 rpm, respectively, in order to improve homogeneity. La3Ni2O6 was obtained from the La3Ni2O7 single crystals by the topochemical reduction method. La3Ni2O7 single crystals were enclosed in an aluminum foil and sealed in vacuum with stoichiometric CaH2 powders. The reaction was under the condition of 300 ∘C for 4 days.
X-ray photoemission spectroscopy (XPS) measurements was carried out on an XPS machine (Escalab 250 Xi, Thermo Fisher). Argon sputtering was adopted to remove the surface contamination. The sputtering depth was about 100 nm. The monochromatic Al Kα radiation with photon energy of 1486.6 eV was applied to analyses the valent states of nickel. The X-ray beam was focused on a 0.5 mm spot surface. In order to obtain high-resolution spectra, the electron energy analyzer was operated at a pass energy of 30 eV. The C 1 photoelectron line (284.8 eV) was used to calibrate the binding energies of the photoelectrons.
Magnetic susceptibility, resistivity, and specific heat measurements were performed on a physical property measurement system (PPMS, Quantum Design). In situ high-pressure electrical resistance measurements were carried out in a diamond anvil cell made from a Be-Cu alloy using a standard four-probe techniqueCai et al. (2020); Sun et al. (2021). NaCl powders were employed as the pressure transmitting medium. The pressure in the resistance measurements was calibrated by the ruby fluorescence shift at room temperature. X-ray single crystal diffraction (XRD) was performed on a single-crystal X-ray diffractometer ( SuperNova, Rigaku) using the Mo-Kα radiation at 300 K. The diffraction data were refined by the Rietveld methodRietveld (1969). Energy-dispersive X-ray spectroscopy (EDS) (EVO, Zeiss) was employed to determine the compositions of the crystals. In addition, Laue X-ray diffraction technique was utilized to confirm the crystal orientation and single crystallinity.
| Empirical formula | La3Ni2O7 | La3Ni2O6 |
|---|---|---|
| Space group | Cmcm (Orthorhombic) | I4/mmm (Tetragonal) |
| Unit-cell parameters | Å; Å | Å |
| Å | Å | |
| La1 | ||
| La2 | ||
| Ni | ||
| O1 | ||
| O2 | ||
| O3 | ||
| O4 | ||
| Goodness-of-fit on | 1.184 | 0.966 |
| Final indexes (all data) | =0.0327 | =0.0469 |
III Results
III.1 La3Ni2O7
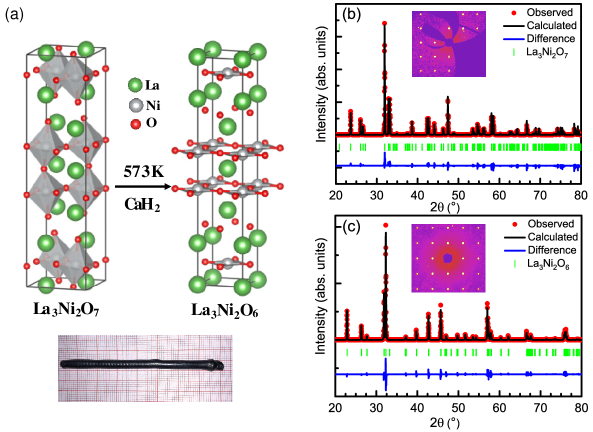
Crystal structures of La3Ni2O7 and La3Ni2O6 are shown in Fig. 1(a). La3Ni2O7 crystallizes in orthorhombic symmetry (space group: Cmcm) with distorted vertex-sharing NiO6 octahedra and rock-salt La-O layersLing et al. (1999). The structure can be termed as inter-growth of two NiO6 octahedra planes and a La-O fluorite-type layer, stacking along the direction. La3Ni2O6 with the apical oxygen atoms removed crystallizes in tetragonal symmetry (space group: I4/mmm). The structure is stacked by two corner-sharing square NiO2 planes and a La-O fluorite-type layer along the directionPoltavets et al. (2006). The XRD patterns measured on single crystals reveal high quality of our samples [Fig. 1(b) and 1(c)]. Detailed Rietveld-refined structural results are summarized in Table 2. The compositions of the single crystals determined by EDS are La2.95Ni2O7 and La2.92Ni2O6, respectively, close to the stoichiometric compositions within the instrumental accuracy. We note the oxygen content is not sensitive in EDS and the contents of La have been normalized by that of Ni.
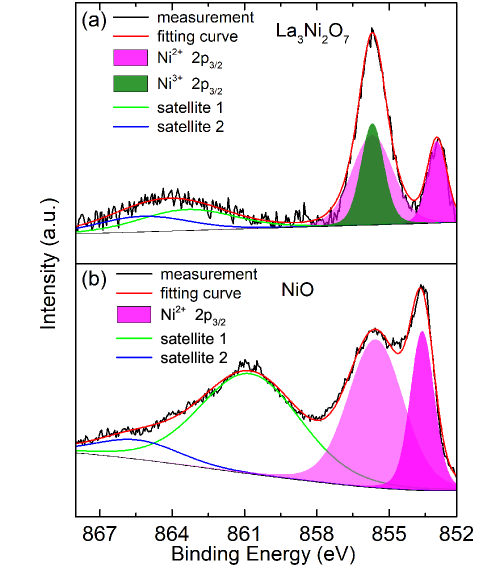
The valent states of Ni are crucial for realization of charge and spin order. La3Ni2O7 is metallic, where nickel ions could host a valence of +2.5, or a mixed-valent states of +2 and +3. Figure 2 (a) shows the XPS spectrum of Ni ions in La3Ni2O7. As a comparison, an XPS spectrum of Ni2+ measured on NiO is presented in Fig. 2 (b). The main peaks of the spectrum of Ni2+ locate at 853.6 and 855.6 eV. In addition, a broad satellite peak appears at a higher binding energy of 860.8 eV. These features are typical for nickel ions with the divalent oxidation state. The XPS spectrum of La3Ni2O7 exhibits asymmetric doublet peaks at 853.0 and 855.7 eV as shown in Fig. 2 (a). We preserve the ratio of the two peaks of Ni2+ as measured on NiO and fit the spectrum of La3Ni2O7. A peak at 855.7 eV could be separated, yielding the existence of the trivalent oxidation stateQiao and Bi (2011); Liu et al. (2019); Chen et al. (2019). Thus, our XPS measurements reveal that the valent states of nickel in La3Ni2O7 are a mixture of the divalent Ni2+ and trivalent Ni3+ oxidation states.
Figure 3(a) shows the temperature dependence of the resistance for La3Ni2O7 single crystals, revealing a metallic ground state that is analogous to the , and RP compoundsZhang et al. (1994); Guo et al. (2018); Rivadulla et al. (2003); Liu et al. (2020). We observe two anomalies in resistance, one at 110 K and the other one at 153 K. They could be identified unambiguously in the deviation of resistance against temperature as shown in Fig. 3 (a). The former one at 110 K is close to the anomalies observed in Hall and Seebeck coefficientsSreedhar et al. (1994); Taniguchi et al. (1995); Kobayashi et al. (1996). While the later one at 153 K is weaker and has not be identified in the previous measurements on powder samples. For LaNiO3 and La4Ni3O10, a metal-metal transition near 150 K has been observed in resistivity, where the origin has been proved to result from intertwined charge and spin density waveGuo et al. (2018); Zhang et al. (2020c).
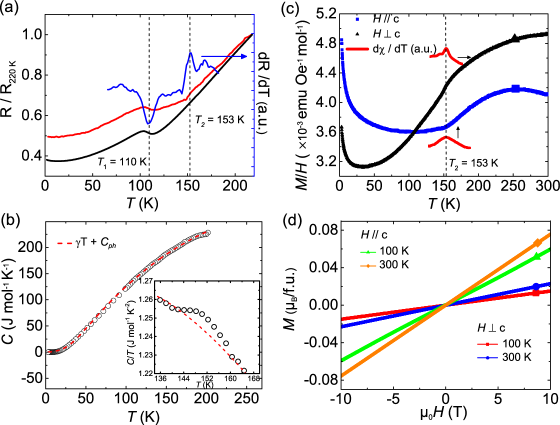
To investigate the origin of the transitions in La3Ni2O7, we measured the specific heat and magnetization against temperature and magnetic field. The specific heat for temperatures between 3 and 200 K is shown in Fig. 3 (b). A model C = T + Cph was employed to fit the data, where T and Cph represent the contributions of electrons and phonons, respectively. A modified Debye model considering the existence of two phonon modes that reconcile the heavy atoms (La and Ni) and light atoms (O) was considered to describe the phonon contribution, , where Jmol-1K-1 is the ideal gas constant and Cn represents the numbers of the heavy or light atoms in a formula unit. The modeling reveals that, of the 12 atoms in the formula unit, 5 atoms have a Debye temperature of K and 7 atoms have a Debye temperature of KLi et al. (2020b); Ortega-San Martin et al. (2006).The fitting also yields = 7.3 mJmol-1K-2, close to the value of 6.4 mJmol-1K-2 revealed from powder samplesWu et al. (2001). With the estimated densities of charge-carriers and mass of the free-electrons, the value of electron effective mass m∗/m0 of La3Ni2O7 is 2.12, reminiscent of 2.56 for La4Ni3O10 and much lower than 15 for LaNiO3Rajeev et al. (1991); Sreedhar et al. (1994); Zhang and Greenblatt (1995). The effective mass of electrons suggests that the electronic correlations in La3Ni2O7 and La4Ni3O10 are weaker than that of the n= compound LaNiO3. The anomaly at 153 K could also be observed in specific heat, as shown in the inset of Fig. 3 (b), in consistence with the temperature of the anomaly at 153 K in resistance.
Figure 3 (c) displays temperature dependence of the magnetization of La3Ni2O7 single crystals at 4000 Oe. The kink at 153 K for both and could be identified in magnetization and , suggesting a same physical origin as the anomalies at the identical temperature in resistance and specific heat. While the anomaly at 110 K in resistance does not show in magnetization. The upturn below 50 K with decreasing temperature in Fig. 3 (c) may be related to magnetic impurities or lattice imperfections. We note the magnetization of La3Ni2O7 single crystals shown in Fig. 3 (c) is reminiscent of that observed in LaNiO3 and La4Ni3O10 single crystalsGuo et al. (2018); Zhang et al. (2020c). For the later two materials, intertwined charge and spin density wave has been confirmed by neutron and X-ray diffraction measurements. The magnetization as a function of magnetic field and temperature is shown in Fig. 3 (d). The magnetization evolutes linearly against magnetic field up to T, indicating antiferromagnetic correlations. In the scenario of spin order, the anisotropy of the magnetization in Fig. 3 (d) suggests the moment of Ni aligned in-plane.
III.2 La3Ni2O6
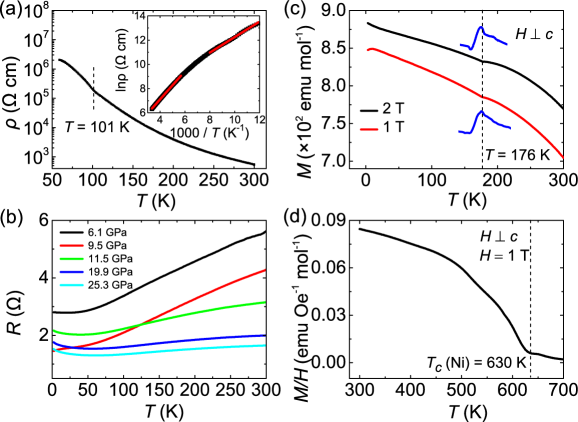
The ground state of La3Ni2O6 with mixed Ni and Ni was predicted to be an AF insulator with a checkerboard charge order and both the AF and ferromagnetic (FM) interactions. Pressure will suppress the FM interactions further and may induce superconductivityBotana et al. (2016). However, only was the insulating state confirmed due to the limitation of powder samplesPoltavets et al. (2009). We obtain single crystals of La3Ni2O6 from topochemical reduction of the La3Ni2O7 single crystals. Temperature dependence of the electrical resistivity is shown in Fig. 4 (a) and the inset. The semiconducting behavior is analogous to the measurements on powder samplesPoltavets et al. (2009). The resistivity of La3Ni2O6 displays a weak kink at 101 K, in contrast to the abrupt change in resistivity at 100 K for La4Ni3O8 that corresponds to the formation of charge and spin stripe orderZhang et al. (2016, 2019). By fitting the resistivity to the activation-energy model exp, two thermal activation energy gaps of and 100 meV are obtained, corresponding to K and K, respectively. To explore superconductivity in La3Ni2O6, the resistance measured under pressure is shown in Fig. 4 (b). The metallization is achieved at 6.1 GPa. However, no superconductivity emerges in our samples up to 25.3 GPa.
Figure 4 (c) displays the magnetization of La3Ni2O6 with the magnetic field perpendicular to the axis. A kink at 176 K could be identified in the derivative of magnetization for the magnetic fields at 1 and 2 T. As theoretical suggestions, the kink on magnetization at 176 K may be associated with the charge and spin order of La3Ni2O6Botana et al. (2016), which may also result in the change of the fitted thermal activation gaps against temperature as shown in the inset of Fig. 4 (a). The magnetization decreases as the temperature increasing, similar to the behavior of a ferromagnet. It is known that the topochemical reduction method would induce Ni impurityLi et al. (2020a). A high-temperature magnetization measurement was conducted, revealing a FM transition at K, as shown in Fig. 4 (d). The result demonstrates the existence of Ni impurity.
IV Discussion and Summary
The mixed-valent and spin states of Ni in the ternary La-Ni-O system tend to form charge and spin order. The emergence of the two types of order has been verified in hole doped La2-xSrxNiO4 (1/4, 1/3, and 1/2), La4Ni3O10, and La4Ni3O8Chen et al. (1993); Lee and Cheong (1997); Zhong et al. (2017); Zhang et al. (2016, 2019). For the trilayer RP compounds, the ratio of the magnetic Ni3+ () and nonmagnetic Ni2+ () is ; for the bilayer RP compounds, the value of this ratio drops to . The formation of charge order is expected, while the spin order may be weaker in the bilayer compounds. The changes in resistivity, susceptibility, and specific heat at 153 K are indeed less pronounced in La3Ni2O7 single crystals compared to that of La4Ni3O10. The possibility that the charge order emerges in the bilayer La-Ni-O system without accompanying by the spin order could not be ruled out. In this case, the anomaly in magnetization could be due to charge-spin interactions. Our Raman scattering measurements on La3Ni2O7 also reveal an anomaly at 150 K for the position of the peak at 597 cm-1 (data not shown). Based on previous studies, the anomaly in resistance at 110 K may be related to the change of carrier concentration induced by a structural evolution against temperatureSreedhar et al. (1994); Zhang et al. (1994); Taniguchi et al. (1995); Kobayashi et al. (1996); Ling et al. (1999).
For La3Ni2O6, an insulating ground state with a checkerboard charge order and AF order based on Ni1+ is expectedBotana et al. (2016). The average valence of Ni, +1.5, is close to +1.2 that of the superconducting film samples. Evidences for the charge and spin order have been observed. However, superconductivity does not appear up to 25.3 GPa, where the samples have been metallizedPoltavets et al. (2009). The superconductivity seems to be sensitive to the average-valent state of nickel. In addition, the possible charge and spin order in La3Ni2O6 may suppress superconductivity under pressure. Further synchrotron X-ray and neutron diffraction studies on single crystal samples of the bilayer compounds are necessary to demonstrate the charge and spin order.
In summary, we have grown the bilayer nickelates La3Ni2O7 and La3Ni2O6 single crystals successfully by the floating-zone method with high pressure of oxygen. The structural, magnetic, electronic, and specific heat properties of both compounds are characterized in detail. Electronic measurements show metallic property for La3Ni2O7, and semiconducting for La3Ni2O6 with a thermal activation gap of 55 meV. The resistance, magnetization, and specific heat all reveal a transition-like anomaly at 153 K for La3Ni2O7, suggesting the formation of charge and spin order. In addition, the magnetization of La3Ni2O6 also yields a kink at 176 K, reminiscent to the charge and spin order in La3Ni2O7. Pressure above 6.1 GPa could metallize La3Ni2O6. However, no superconductivity is observed up to 25.3 GPa. Our data suggest the formation of charge and spin order may be a universal characteristic for nickelates with mixed-valent states of nickel.
V Acknowledgments
Work was supported by the National Natural Science Foundation of China (Grants No. 12174454, 11904414, 11904416, U2130101), the Guangdong Basic and Applied Basic Research Foundation (No. 2021B1515120015), and National Key Research and Development Program of China (No. 2019YFA0705702, 2020YFA0406003, and 2021YFA1400401, 2021YFA0718900).
References
- Li et al. (2019) D. Li, K. Lee, B. Y. Wang, M. Osada, S. Crossley, H. R. Lee, Y. Cui, Y. Hikita, and H. Y. Hwang, Nature 572, 624 (2019).
- Vignolle et al. (2007) B. Vignolle, S. Hayden, D. McMorrow, H. M. Rønnow, B. Lake, C. Frost, and T. Perring, Nature Physics 3, 163 (2007).
- Keimer et al. (2015) B. Keimer, S. A. Kivelson, M. R. Norman, S. Uchida, and J. Zaanen, Nature 518, 179 (2015).
- Zhang et al. (2020a) G.-M. Zhang, Y.-f. Yang, and F.-C. Zhang, Physical Review B 101, 020501 (2020a).
- Zhang et al. (2020b) Y. Zhang, L.-F. Lin, W. Hu, A. Moreo, S. Dong, and E. Dagotto, Physical Review B 102, 195117 (2020b).
- Wang et al. (2020) B.-X. Wang, H. Zheng, E. Krivyakina, O. Chmaissem, P. P. Lopes, J. W. Lynn, L. C. Gallington, Y. Ren, S. Rosenkranz, J. Mitchell, et al., Physical Review Materials 4, 084409 (2020).
- Lee and Pickett (2004) K.-W. Lee and W. Pickett, Physical Review B 70, 165109 (2004).
- Anisimov et al. (1999) V. Anisimov, D. Bukhvalov, and T. Rice, Physical Review B 59, 7901 (1999).
- Gu and Wen (2022) Q. Gu and H. H. Wen, The Innovation 3, 100202 (2022).
- Pan et al. (2022) G. A. Pan, D. Ferenc Segedin, H. LaBollita, Q. Song, E. M. Nica, B. H. Goodge, A. T. Pierce, S. Doyle, S. Novakov, D. Córdova Carrizales, et al., Nature Materials 21, 160 (2022).
- Wang et al. (2021) N. N. Wang, M. W. Yang, K. Y. Chen, Z. Yang, H. Zhang, Z. H. Zhu, Y. Uwatoko, X. L. Dong, K. J. Jin, J. P. Sun, et al., arXiv:2109.12811 (2021).
- He et al. (2021) C. He, X. Ming, Q. Li, X. Zhu, J. Si, and H.-H. Wen, Journal of Physics: Condensed Matter 33, 265701 (2021).
- Li et al. (2020a) Q. Li, C. He, J. Si, X. Zhu, Y. Zhang, and H.-H. Wen, Communications Materials 1, 16 (2020a).
- Huo et al. (2022) M. Huo, Z. Liu, H. Sun, L. Li, H. Liu, C. Huang, F. Liang, B. Shen, and M. Wang, arXiv:2204.08050 (2022).
- Guo et al. (2018) H. Guo, Z. Li, L. Zhao, Z. Hu, C. Chang, C.-Y. Kuo, W. Schmidt, A. Piovano, T. Pi, O. Sobolev, et al., Nature Communications 9, 43 (2018).
- Zhang et al. (2020c) J. Zhang, D. Phelan, A. Botana, Y.-S. Chen, H. Zheng, M. Krogstad, S. G. Wang, Y. Qiu, J. Rodriguez-Rivera, R. Osborn, et al., Nature Communications 11, 6003 (2020c).
- Garc´ıa-Muñoz et al. (1992) J. García-Muñoz, J. Rodríguez-Carvajal, P. Lacorre, and J. Torrance, Physical Review B 46, 4414 (1992).
- Zhang et al. (2016) J. Zhang, Y.-S. Chen, D. Phelan, H. Zheng, M. Norman, and J. Mitchell, Proceedings of the National Academy of Sciences 113, 8945 (2016).
- Zhang et al. (2019) J. Zhang, D. M. Pajerowski, A. S. Botana, H. Zheng, L. Harriger, J. Rodriguez-Rivera, J. P. Ruff, N. Schreiber, B. Wang, Y.-S. Chen, et al., Physical Review Letters 122, 247201 (2019).
- Sreedhar et al. (1994) K. Sreedhar, M. McElfresh, D. Perry, D. Kim, P. Metcalf, and J. Honig, Journal of Solid State Chemistry 110, 208 (1994).
- Zhang et al. (1994) Z. Zhang, M. Greenblatt, and J. Goodenough, Journal of Solid State Chemistry 108, 402 (1994).
- Taniguchi et al. (1995) S. Taniguchi, T. Nishikawa, Y. Yasui, Y. Kobayashi, J. Takeda, S. ichi Shamoto, and M. Sato, Journal of the Physical Society of Japan 64, 1644 (1995).
- Kobayashi et al. (1996) Y. Kobayashi, S. Taniguchi, M. Kasai, M. Sato, T. Nishioka, and M. Kontani, Journal of the Physical Society of Japan 65, 3978 (1996).
- Ling et al. (1999) C. D. Ling, D. N. Argyriou, G. Wu, and J. J. Neumeier, Journal of Solid State Chemistry 152, 517 (1999).
- Zhang and Greenblatt (1995) Z. Zhang and M. Greenblatt, Journal of Solid State Chemistry 117, 236 (1995).
- Wu et al. (2001) G. Wu, J. Neumeier, and M. Hundley, Physical Review B 63, 245120 (2001).
- Seo et al. (1996) D.-K. Seo, W. Liang, M.-H. Whangbo, Z. Zhang, and M. Greenblatt, Inorganic chemistry 35, 6396 (1996).
- Botana et al. (2016) A. S. Botana, V. Pardo, W. E. Pickett, and M. R. Norman, Physical Review B 94, 081105 (2016).
- Poltavets et al. (2009) V. V. Poltavets, M. Greenblatt, G. H. Fecher, and C. Felser, Physical Review Letters 102, 046405 (2009).
- Crocker et al. (2013) J. Crocker, A. Dioguardi, K. Shirer, V. Poltavets, M. Greenblatt, P. Klavins, N. Curro, et al., Physical Review B 88, 075124 (2013).
- Zhang et al. (2020d) J. Zhang, H. Zheng, Y.-S. Chen, Y. Ren, M. Yonemura, A. Huq, and J. Mitchell, Physical Review Materials 4, 083402 (2020d).
- Cai et al. (2020) W. Cai, H. Sun, W. Xia, C. Wu, Y. Liu, H. Liu, Y. Gong, D.-X. Yao, Y. Guo, and M. Wang, Physical Review B 102, 144525 (2020).
- Sun et al. (2021) H. Sun, C. Chen, Y. Hou, W. Wang, Y. Gong, and M. Huo, Science China: Physics, Mechanics and Astronomy 64, 118211 (2021).
- Rietveld (1969) H. M. Rietveld, J. Appl. Cryst. 2, 65 (1969).
- Poltavets et al. (2006) V. V. Poltavets, K. A. Lokshin, S. Dikmen, M. Croft, T. Egami, and M. Greenblatt, Journal of the American Chemical Society 128, 9050 (2006).
- Qiao and Bi (2011) L. Qiao and X. Bi, Epl 93, 57002 (2011).
- Liu et al. (2019) Y. Liu, P. Liu, W. Qin, X. Wu, and G. Yang, Electrochimica Acta 297, 623 (2019).
- Chen et al. (2019) H. Chen, Z. Wu, Y. Zhong, T. Chen, X. Liu, J. Qu, W. Xiang, J. Li, X. Chen, X. Guo, et al., Electrochimica Acta 308, 64 (2019).
- Rivadulla et al. (2003) F. Rivadulla, J.-S. Zhou, and J. Goodenough, Physical Review B 67, 165110 (2003).
- Liu et al. (2020) C. Liu, V. F. Humbert, T. M. Bretz-Sullivan, G. Wang, D. Hong, F. Wrobel, J. Zhang, J. D. Hoffman, J. E. Pearson, J. S. Jiang, et al., Nature Communications 11, 1402 (2020).
- Li et al. (2020b) L. Li, N. Narayanan, S. Jin, J. Yu, Z. Liu, H. Sun, C.-W. Wang, V. K. Peterson, Y. Liu, S. Danilkin, et al., Physical Review B 102, 094413 (2020b).
- Ortega-San Martin et al. (2006) L. Ortega-San Martin, J. P. Chapman, L. Lezama, J. Sánchez Marcos, J. Rodríguez-Fernández, M. I. Arriortua, and T. Rojo, European Journal of Inorganic Chemistry 2006, 1362 (2006).
- Rajeev et al. (1991) K. Rajeev, G. Shivashankar, and A. Raychaudhuri, Solid State Communications 79, 591 (1991).
- Chen et al. (1993) C. H. Chen, S.-W. Cheng, and A. S. Cooper, Physical Review Letters 71, 2461 (1993).
- Lee and Cheong (1997) S. H. Lee and S.-W. Cheong, Physical Review Letters 79, 2514 (1997).
- Zhong et al. (2017) R. Zhong, B. L. Winn, G. Gu, D. Reznik, and J. M. Tranquada, Physical Review Letters 118, 177601 (2017).