Supplementary \alsoaffiliationCentro de Física de Materiales MPC (CSIC/UPV-EHU), 20018 Donostia-San Sebastián, Spain \alsoaffiliationIkerbasque, Basque Foundation for Science, 48013 Bilbao, Spain \alsoaffiliationIkerbasque, Basque Foundation for Science, 48013 Bilbao, Spain
From Solution-to-Surface: Persistence of the Diradical Character of a Diindenoanthracene Derivative on a Metallic Substrate
Abstract
Organic diradicals are envisioned as elementary building blocks for designing a new generation of spintronic devices and have been used in constructing prototypical field effect transistors and non-linear optical devices. Open-shell systems, however, are also reactive, thus requiring design strategies to “protect” their radical character from the environment, especially when they are embedded into solid-state devices. Here, we report the persistence on a metallic surface of the diradical character of a diindeno[b,i]anthracene (DIAn) core protected by bulky end-groups. Our scanning tunneling spectroscopy measurements on single-molecules detected singlet-triplet excitations that were absent for DIAn species packed in assembled structures. Density functional theory simulations unravel that molecular geometry on the metal substrate can crucially modify the value of the single-triplet gap via the delocalization of the radical sites. The persistence of the diradical character over metallic substrates is a promising finding for integrating radical-based materials in functional devices.
![[Uncaptioned image]](https://cdn.awesomepapers.org/papers/898d84ec-8992-4751-8edf-af30bf41d2b7/x1.png)
Diradical molecules are promising candidates for the design of new generation nonlinear optic (NLO) 1 and spintronic devices 2, 3. As examples, diradicals have been used to build spin filters 4, field-effect transistors5, and they could potentially be used for memory storage devices 1, 3. In recent years, much focus has been directed to the understanding of the interactions established between the two spin centers of a diradical, and how those interactions can be controlled 1, 6, 7, 8.
To date, the impact of a contact metal electrode on the electronic and magnetic properties of diradicals remains unclear, especially since the influence of molecular geometry, adsorption, and packing has yet to be revealed. Those parameters have been shown to play a crucial role in defining the magnetic properties of metal-organic molecules on surfaces 9, 10, 11, 12, 13, 14, and are also expected to affect the properties of purely organic diradicals. Most of the recent studies on diradicals are performed in soft solution or solid environments. Therefore, exploring their structures on metallic surfaces can be of great significance to disentangle remaining unknown questions about these fascinating molecules.
Among all diradical molecules, the diindeno[b,i]anthracene (DIAn) framework is of particular interest due to its high solubility in common organic solvents, high thermal stability, ease of sublimation, and excellent oxidation resistance 15. DIAn has also exceptional electronic properties, such as a small singlet-triplet gap and a balanced ambipolar charge-transport behavior 16, 17, 18, 19. Owing to these properties, DIAn molecules are perfectly suitable for mass production for future market applications in, for instance, high-performance organic field-effect transistors 5. In a more fundamental scenario, DIAn is ideally suited to investigate, at the atomic scale, the magnetic behavior of prototypical diradical molecules in contact with inorganic interfaces, seeking strategies to maintain their molecular functionality when forming part of solid state devices.
Here we demonstrate that individual DIAn molecules maintain their diradical character on a metallic substrate, with a reduced singlet-triplet gap compared to values observed in the solid state. We studied DIAn molecules and assemblies adsorbed on an atomically clean Au(111) substrate, and probed inter- and intra-molecular spin interactions by scanning tunneling spectroscopy (STS). Using the Kondo effect as a fingerprint of molecular magnetism, 20, 21, 22, 23, 24, 25, 26 we probe local spin interactions inside the diradicals, unraveling underlying mechanisms at the origin of magnetic interactions between the two spin centers of the diradical. More importantly, we demonstrate that those magnetic interactions depend on molecular conformation over the surface and on their interaction with other species in assembled nanostructures. In particular, supported by density functional theory (DFT) simulations we show that the rotation of mesityl substituents modifies the magnetic interaction between the two spin centers of a single DIAn molecule. These results outline the relevance of intermolecular interactions in supramolecular nanoarchitectures in the magnetic properties of open-shell molecular systems.
The core of the DIAn molecule is composed of two indene groups (black section, Fig. 1a), spaced by an anthracene bridge (red section in Fig. 1a). The indene moieties endow DIAn with the radical character of the molecule 15 and, here, incorporate bulky mesityl substituents (green section in Fig. 1a) for protecting them. Additionally, (triisopropylsilyl)ethynyl groups (CCSi(i-Pr)3, orange section, Fig. 1a) are attached to the quinoidal anthracene core to provide high stability against oxidation and high solubility. Details of the synthesis of DIAn have been reported previously 15.
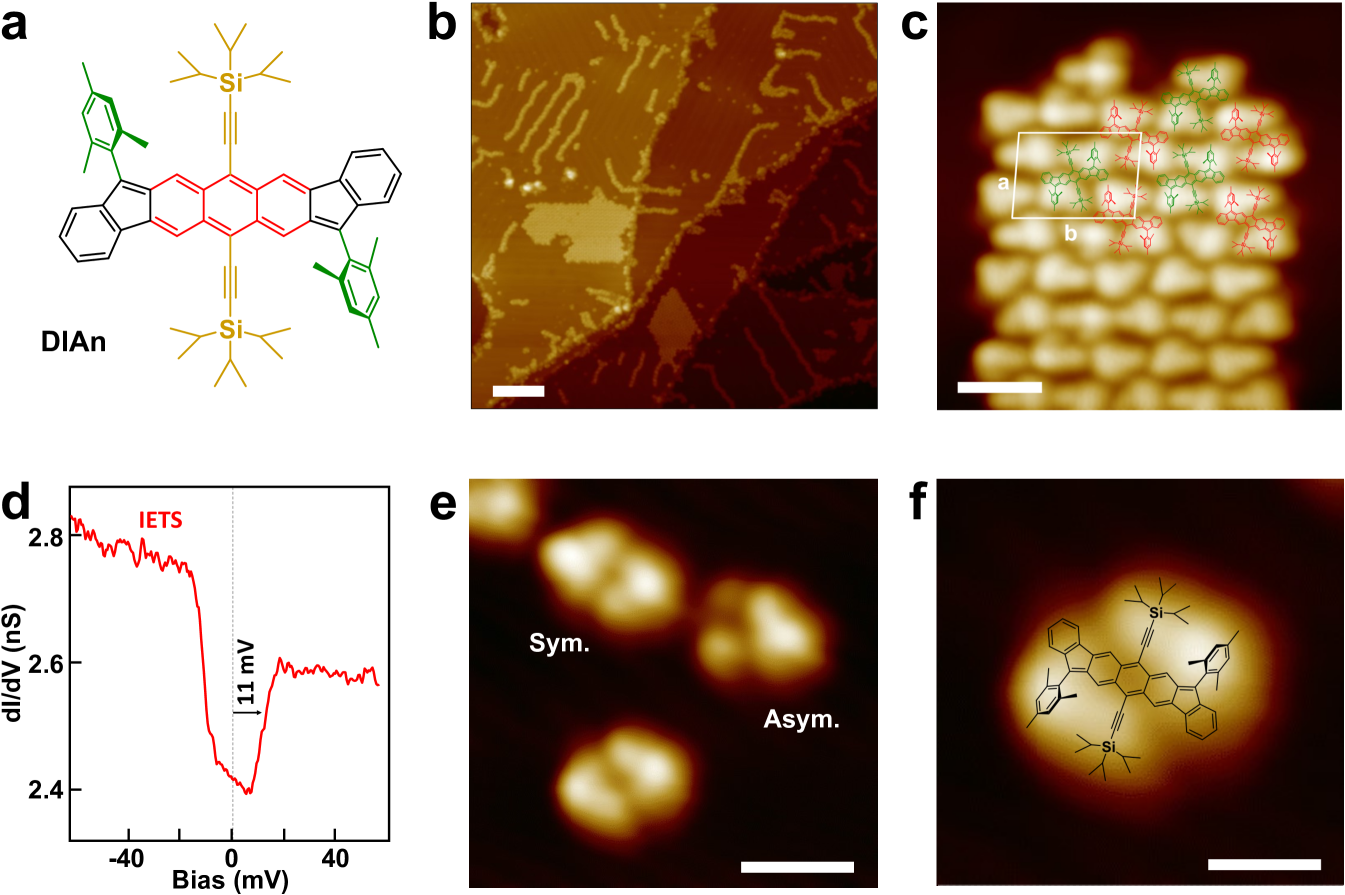
We deposited DIAn diradicals onto an atomically flat Au(111) surface by thermal sublimation of the molecular powder in ultrahigh vacuum at a temperature of 563 K. Scanning tunneling microscopy (STM) images at 4 K show that DIAn diradicals assemble in two types of structures for sub-monolayer coverages: extended close-packed domains and molecular chains (Fig. 1b). The former consists of a parallelogram network with a unit cell (white box in Fig. 1c) containing two DIAn molecules, as indicated in the molecular model in Fig. 1c. Differential conductance () spectra on the molecules in these close-packed domains show two bias-symmetric steps at 11 meV (Fig. 1d). The spectral feature is highly localized at the central anthracene core and absent over the protruding groups. We tentatively attribute it to the inelastic excitation of a molecular vibrational mode 27, 28. In fact, Raman spectra of DIAn crystals show a prominent band around 11 meV corresponding to the out-of-phane deformation of the mesityl and of the isopropylsilyl groups (Figs. S1, S2). Molecular chains, in contrast to the domains, are rather disordered structures, weakly packed, and oriented along the Au(111) herringbone reconstruction.
To explore single DIAn diradicals, we moved apart individual molecules from the chain structures using the tip of the STM. As in the domains, DIAn molecules appear in STM images as two inverted protrusions, each composed of the bulkier mesityl groups (Mes) and the lower (triisopropylsilyl)ethynyl moiety (CCSi(i-Pr)3), as shown in Figs. 1e-f. It is important to note that, in some occasions, tip-manipulated DIAn molecules appeared with an asymmetric configuration, with one half appearing with lower height (top right of Fig. 1e). Although we cannot state the precise origin of this different aspect, we speculate that it might be due to a distortion (e.g. a rotation) of the mesityl groups.
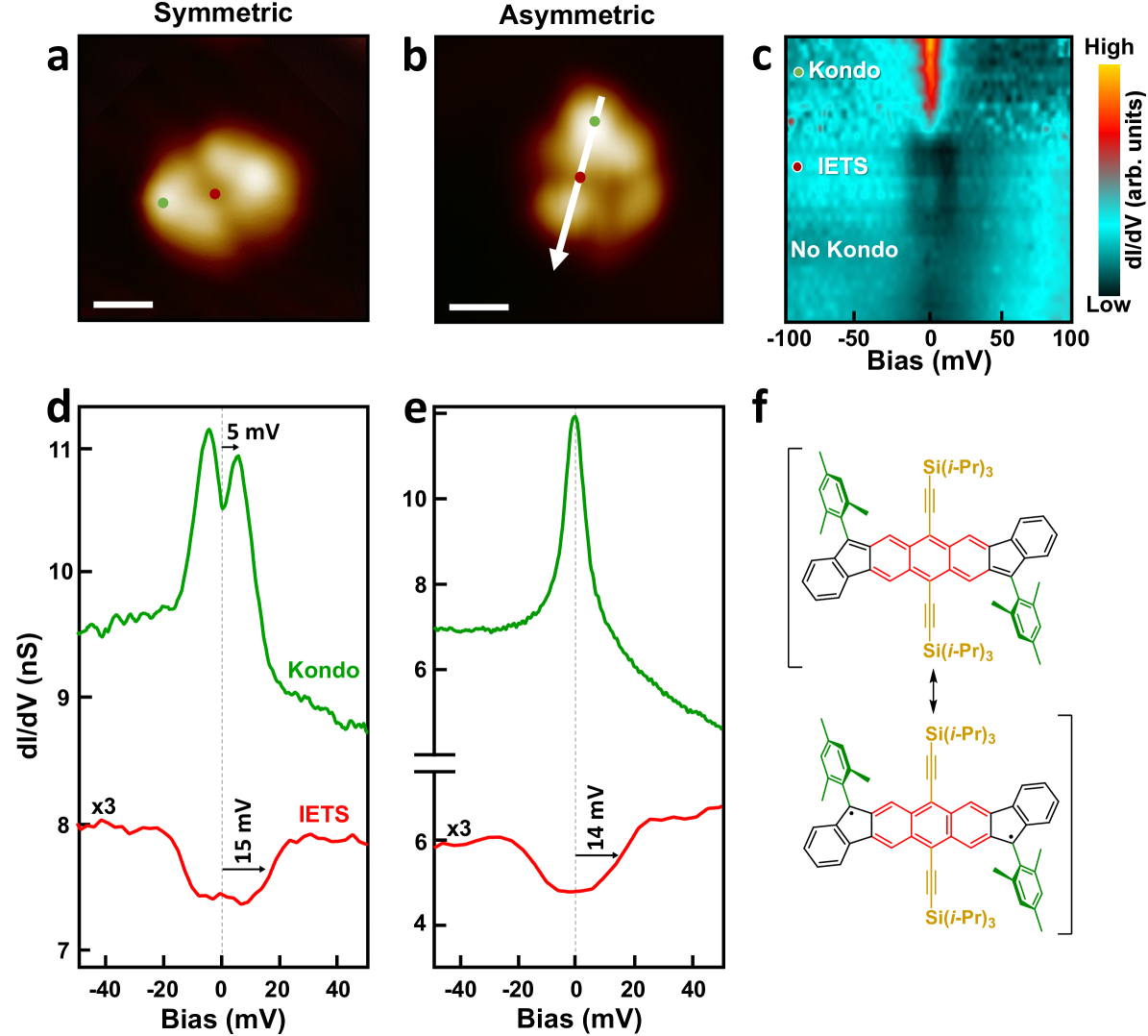
The open-shell character of the isolated DIAn species could be resolved via STS measurements. Differential conductance () spectra over their mesityl groups (Fig. 2a) resolved now two narrow peaks centered symmetrically around zero bias (green curve in Fig. 2d), which reveal the survival of their diradical state on the Au(111) substrate. Owing to a weak interaction of the radical state with the metal surface, its associated spin S=1/2 can be partially screened via the Kondo effect 29, 20, 30, 10, 31, resulting in a narrow logarithmic resonance at zero bias. However, over DIAn’s bulky sides, we observed, instead, a double-peak structure. This is attributed to the presence of two antiferromagnetically interacting radical states. As shown in Fig. 2f, DIAn can lie either in an open shell non-Kekulé structure or in a closed shell state. In the former, two radicals appear localized at the junctions between the indene and mesityl groups (i.e., at the apical carbons of the five-membered rings), spaced by the central anthracene bridge, which mediates their antiferromagnetic interaction between them, forming a singlet ground state. In this configuration, the lower dI/dV signal at zero-bias reflects the absence of Kondo states for the singlet ground state. At tunneling energies equal to or larger than , dI/dV peaks reflect the excitation of a triplet state, with their corresponding energy providing a direct measure of 22, 32. Above these onsets, Kondo-like dynamical fluctuations in the triplet state, now accessible by inelastic tunneling electrons, results in the logarithmic tail and the appearance of a split-Kondo resonance rather than inelastic steps 33, 34.
In contrast, on the distorted DIAn molecules, a single dI/dV peak centered at zero bias is measured only over the brighter mesityl group (green curve, Fig. 2e), while a featureless spectrum appears on the lower half of the molecule ( stacked plot, Fig. 2c). This reveals that on the distorted side, the radical state is either quenched probably due to a conformational change or a strong interaction with the metal underneath. Additionally, bias-symmetric dI/dV steps at meV were detected over the central anthracene core on both pristine and distorted DIAn species (red curves in Fig. 2d,e). Since we observe them with similar intensity on the diradical and monoradical species, we can safely exclude their magnetic origin. Instead, we tentatively assign them to the excitation of the vibrational mode observed on the assembled domains, here at slightly higher energy probably due to the different conformation adopted when isolated on the metal substrate.
The shifted Kondo resonances on DIAn molecules amount to a singlet-triplet excitation energy of 5 meV. This value is significantly smaller than the singlet-triplet energy difference obtained from density functional theory (DFT) simulations of DIAn in the gas phase 15. In particular, our DFT results in Figs. S3a and S3b corroborate the singlet ground state, and find a parallel spin configuration at almost 140 meV above, signaling for a singlet-triplet gap much larger than in our experiment, and similar to previous measurements of DIAn 17.
The origin of the smaller inter-radical exchange interaction observed in the experiment is likely connected with molecular modifications when adsorbed onto the gold surface. We have simulated the geometric relaxation and magnetic state of a DIAn molecule on a Au(111) slab using DFT (results summarized in Figs. S3-S7). The first important result is that DFT reproduces the survival of the diradical character of DIAn molecules on the gold surface (Fig. S3g). The molecule is physisorbed and keeps C-Au distances larger than 3 Å. After geometry optimizations, two magnetic configurations were converged corresponding to a ground state and an () excited state, with an energy spacing significantly smaller than in the gas phase. Thus, DFT confirms that, on the gold surface, the exchange interaction between indene radicals is reduced. The metallic substrate imposes, first, a partial planarization on the molecular structure by rotating the mesityl groups (Mes) towards the diindeno anthracene plane. As shown in Fig. S4, the mesityl rotation induces a slight delocalization of the radical towards their aromatic center, which effectively increases the separation between spin centers and, consequently, reduces their magnetic interaction. Additional corrections to the simulations due to Coulomb correlations (considered in Fig. S6), or the renormalization of exchange coupling due to Kondo correlations 35 can account for the further reduction of the reduced exchange observed in our experiments.
Our findings demonstrate that the commonly assumed inert role of molecular substituents 15 (e.g. Mes, CCSi(i-Pr)3) on the active magnetic properties of diindeno[b,i]anthracene derivatives needs to be revised when the diradicals are put in contact with a metallic electrode. Although, unpaired electron spins remain present in the molecule, their interaction is altered significantly upon adsorption on a metallic electrode. We found that the CCSi(i-Pr)3 group connected at the anthracene core allows preserving the spin polarization of isolated DIAn diradicals on gold. Fig. S7 shows that when the two CCSi(i-Pr)3 groups were replaced by CCSiMe3, DIAn molecules are found to adsorb closer to the surface. In that case, the gold substrate imposes a bending of CCSi(i-Pr)3 triple bonds (Fig. S7a-c), while a gold atom is slightly pushed up from the surface plane (Fig. S7a). Such modification of the molecule-substrate separation modifies the charge distribution and quenches spin polarization over the molecular backbone.
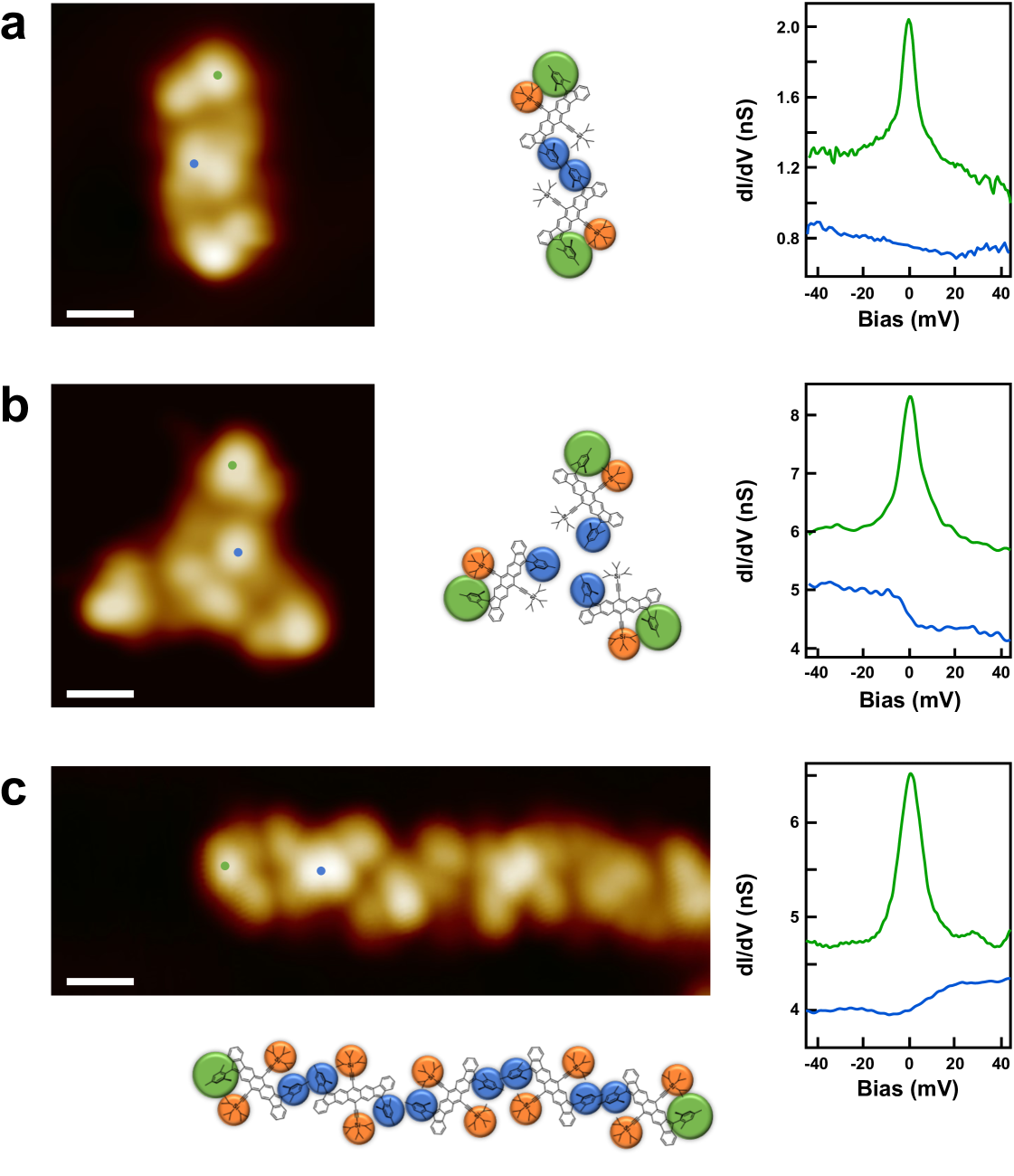
Next, we show that the spin interaction in DIAn diradicals is modified in close-packed nanostructures made of a few molecules. Figure 3 shows nanostructures formed by two, three, or more DIAn molecules. Spectra recorded on the interacting mesityl groups of a DIAn dimer (Figure 3a, blue dot), reveal the absence of a magnetic fingerprint (blue curves), while spectra on the non-interacting mesityl groups (green dot), reveal now a single Kondo feature (green curves). This is further confirmed by spatial maps of the Kondo resonance shown in Fig. S8, which illustrate the localization of the Kondo signal exclusively over external mesityl termini. This observation is consistent across other interacting molecular nanostructures, such as trimers (Figure 3b) and molecular chains (Figure 3c).
The absence of a Kondo resonance on the neighbouring mesityls can be explained caused as a combination of both a finite overlap of their electronic wavefunction of two neighboring spins and the concomitant intramolecular rotation of the involved mesityl groups for the assembly. Nevertheless, the quenching of one of the two radicals of DIAn also explains the observation of a single Kondo resonance on the external mesityl groups in DIAn dimers, trimers, and chains, in clear contrast with the split Kondo feature observed in isolated molecules. This observation also explains the complete quenching of all Kondo signals in extended 2D close-packed domains, as shown earlier (Fig. 1).
In conclusion, here we report the survival of the magnetic state of DIAn diradicals upon their adsorption on a Au(111) surface. We have studied the spin interactions in the molecule through the many-body Kondo effect by performing scanning tunneling spectroscopy measurements and density functional theory calculations. The significant results from this study are (1) the persistence of the diradical singlet ground state upon adsorption; (2) the detection of spin interactions between the two spin centers of a single diradical; (3) the control over inter-molecular spin interactions through the formation of nanostructures by self-assembly or STM tip manipulation; and (4) the demonstration of the substrate influence on the lowering of the singlet-triplet gap of the DIAn diradical by imposing a rotation of its mesityl groups. Importantly, these are crucial questions raised that need to be solved for the future use of diradicals in spintronic and NLO devices, in which the molecules are contacted with metal electrodes. Our study paves the way to this by precise control of the spin interactions in isolated molecules and assembled nanostructures made of diradicals.
1 Supporting Information
The supporting information file is composed of the following:
-
A
Vibrational mode of DIAn,
-
B
Energy Calculation of Singlet-Triplet States in DIAn adsorbed on gold,
-
C
Change in Electronic Structure After Rotation of Mesityl Groups,
-
D
Projected Density of States and Bond Order Modification in the Gas Phase,
-
E
Influence of Coulomb Repulsion U in the HOMO-LUMO gap, Singlet-Triplet Energy and Bond Lengths,
-
F
Influence of Substituent Chemical Composition on the Magnetic Properties of the DIAn Molecule,
-
G
Kondo Map of DIAn Trimers,
-
H
Controlling the Kondo Signature by Lateral Manipulation,
-
I
Comparison of the long-range spectra of molecular chains and domains,
-
J
Correlation between Molecular Orbital positions and Kondo Resonance,
-
K
Methods.
1.1 Notes
The authors declare no competing financial interest.
We gratefully acknowledge financial support from Spanish MCIN/AEI/ 10.13039/501100011033 and the European Regional Development Fund (ERDF) through grants with number PID2019-107338RBC61, PID2020-115406GB-I00, PID2021-127127NB-I00, and CEX2020-001038-M, and from the European Union (EU) through Horizon 2020 (FET-Open project SPRING Grant. no. 863098). J.C. also acknowledges the Junta de Andalucía, Spain (PROYEXCEL-0328), and M.M.H. acknowledges the NSF (CHE-1954389) for financial support.
References
- Fukuda et al. 2015 Fukuda, K.; Nagami, T.; Fujiyoshi, J.-y.; Nakano, M. Interplay between Open-Shell Character, Aromaticity, and Second Hyperpolarizabilities in Indenofluorenes. The Journal of Physical Chemistry A 2015, 119, 10620–10627
- Shil et al. 2015 Shil, S.; Bhattacharya, D.; Misra, A.; Klein, D. J. A high-spin organic diradical as a spin filter. Phys. Chem. Chem. Phys. 2015, 17, 23378–23383
- Tagami and Tsukada 2004 Tagami, K.; Tsukada, M. Spintronic Transport through Polyphenoxyl Radical Molecules. The Journal of Physical Chemistry B 2004, 108, 6441–6444
- Shil et al. 2015 Shil, S.; Bhattacharya, D.; Misra, A.; Klein, D. J. A high-spin organic diradical as a spin filter. Phys. Chem. Chem. Phys. 2015, 17, 23378–23383
- Zong et al. 2022 Zong, C.; Yang, S.; Sun, Y.; Zhang, L.; Hu, J.; Hu, W.; Li, R.; Sun, Z. Isomeric dibenzooctazethrene diradicals for high-performance air-stable organic field-effect transistors. Chem. Sci. 2022, 13, 11442–11447
- Rajca et al. 2007 Rajca, A.; Takahashi, M.; Pink, M.; Spagnol, G.; Rajca, S. Conformationally Constrained, Stable, Triplet Ground State (S = 1) Nitroxide Diradicals. Antiferromagnetic Chains of S = 1 Diradicals. Journal of the American Chemical Society 2007, 129, 10159–10170
- Majzik et al. 2018 Majzik, Z.; Pavliček, N.; Vilas-Varela, M.; Pérez, D.; Moll, N.; Guitián, E.; Meyer, G.; Peña, D.; Gross, L. Studying an antiaromatic polycyclic hydrocarbon adsorbed on different surfaces. Nature Communications 2018, 9, 1198
- Karafiloglou 1989 Karafiloglou, P. The double (or dynamic) spin polarization in diradicals. Journal of Chemical Education 1989, 66, 816
- Saiful et al. 2020 Saiful, I.; Inose, T.; Tanaka, D.; Mishra, P.; Ogawa, T.; Komeda, T. Hybridized Kondo State Formed by Radical Assemblies. The Journal of Physical Chemistry C 2020, 124, 12024–12029
- Zhang et al. 2015 Zhang, Q.; Kuang, G.; Pang, R.; Shi, X.; Lin, N. Switching Molecular Kondo Effect via Supramolecular Interaction. ACS Nano 2015, 9, 12521–12528
- Amokrane et al. 2017 Amokrane, A.; Klyatskaya, S.; Boero, M.; Ruben, M.; Bucher, J.-P. Role of -Radicals in the Spin Connectivity of Clusters and Networks of Tb Double-Decker Single Molecule Magnets. ACS Nano 2017, 11, 10750–10760
- Karan et al. 2016 Karan, S. et al. Spin Manipulation by Creation of Single-Molecule Radical Cations. Phys. Rev. Lett. 2016, 116, 027201
- Sánchez-Grande et al. 2020 Sánchez-Grande, A. et al. Diradical Organic One-Dimensional Polymers Synthesized on a Metallic Surface. Angewandte Chemie International Edition 2020, 59, 17594–17599
- DiLullo et al. 2012 DiLullo, A.; Chang, S.-H.; Baadji, N.; Clark, K.; Klöckner, J.-P.; Prosenc, M.-H.; Sanvito, S.; Wiesendanger, R.; Hoffmann, G.; Hla, S.-W. Molecular Kondo Chain. Nano Letters 2012, 12, 3174–3179
- Rudebusch et al. 2016 Rudebusch, G. E.; Zafra, J. L.; Jorner, K.; Fukuda, K.; Marshall, J. L.; Arrechea-Marcos, I.; Espejo, G. L.; Ponce Ortiz, R.; Gómez-García, C. J.; Zakharov, L. N.; Nakano, M.; Ottosson, H.; Casado, J.; Haley, M. M. Diindeno-fusion of an anthracene as a design strategy for stable organic biradicals. Nature Chemistry 2016, 8, 753–759
- Rudebusch et al. 2016 Rudebusch, G. E.; Espejo, G. L.; Zafra, J. L.; Peña-Alvarez, M.; Spisak, S. N.; Fukuda, K.; Wei, Z.; Nakano, M.; Petrukhina, M. A.; Casado, J.; Haley, M. M. A Biradical Balancing Act: Redox Amphoterism in a Diindenoanthracene Derivative Results from Quinoidal Acceptor and Aromatic Donor Motifs. Journal of the American Chemical Society 2016, 138, 12648–12654
- Dressler et al. 2018 Dressler, J. J.; Teraoka, M.; Espejo, G. L.; Kishi, R.; Takamuku, S.; Gómez-García, C. J.; Zakharov, L. N.; Nakano, M.; Casado, J.; Haley, M. M. Thiophene and its sulfur inhibit indenoindenodibenzothiophene diradicals from low-energy lying thermal triplets. Nature Chemistry 2018, 10, 1134–1140
- Dressler et al. 2020 Dressler, J. J.; Cárdenas Valdivia, A.; Kishi, R.; Rudebusch, G. E.; Ventura, A. M.; Chastain, B. E.; Gómez-García, C. J.; Zakharov, L. N.; Nakano, M.; Casado, J.; Haley, M. M. Diindenoanthracene Diradicaloids Enable Rational, Incremental Tuning of Their Singlet-Triplet Energy Gaps. Chem 2020, 6, 1353–1368
- Hayashi et al. 2020 Hayashi, H.; Barker, J. E.; Cárdenas Valdivia, A.; Kishi, R.; MacMillan, S. N.; Gómez-García, C. J.; Miyauchi, H.; Nakamura, Y.; Nakano, M.; Kato, S.-i.; Haley, M. M.; Casado, J. Monoradicals and Diradicals of Dibenzofluoreno[3,2-b]fluorene Isomers: Mechanisms of Electronic Delocalization. Journal of the American Chemical Society 2020, 142, 20444–20455
- Ternes et al. 2009 Ternes, M.; Heinrich, A. J.; Schneider, W.-D. Spectroscopic manifestations of the Kondo effect on single adatoms. J. Phys.: Condens. Matter 2009, 21, 053001
- Fernández-Torrente et al. 2008 Fernández-Torrente, I.; Franke, K. J.; Pascual, J. I. Vibrational Kondo Effect in Pure Organic Charge-Transfer Assemblies. Phys. Rev. Lett. 2008, 101, 217203
- Li et al. 2019 Li, J.; Sanz, S.; Corso, M.; Choi, D. J.; Peña, D.; Frederiksen, T.; Pascual, J. I. Single spin localization and manipulation in graphene open-shell nanostructures. Nat. Commun. 2019, 10, 200
- Li et al. 2020 Li, J.; Sanz, S.; Castro-Esteban, J.; Vilas-Varela, M.; Friedrich, N.; Frederiksen, T.; Peña, D.; Pascual, J. I. Uncovering the Triplet Ground State of Triangular Graphene Nanoflakes Engineered with Atomic Precision on a Metal Surface. Physical Review Letters 2020, 124, 177201
- Wang et al. 2022 Wang, T.; Sanz, S.; Castro-Esteban, J.; Lawrence, J.; Berdonces-Layunta, A.; Mohammed, M. S. G.; Vilas-Varela, M.; Corso, M.; Peña, D.; Frederiksen, T.; de Oteyza, D. G. Magnetic Interactions Between Radical Pairs in Chiral Graphene Nanoribbons. Nano Letters 2022, 22, 164–171
- Turco et al. 2023 Turco, E.; Bernhardt, A.; Krane, N.; Valenta, L.; Fasel, R.; Juríček, M.; Ruffieux, P. Observation of the Magnetic Ground State of the Two Smallest Triangular Nanographenes. JACS Au 2023, 3, 1358–1364
- Vilas‐Varela et al. 2023 Vilas‐Varela, M. et al. On‐Surface Synthesis and Characterization of a High‐Spin Aza‐[5]‐Triangulene. Angewandte Chemie International Edition 2023, 62, e202307884
- Stipe et al. 1998 Stipe, B. C.; Rezaei, M. A.; Ho, W. Single-Molecule Vibrational Spectroscopy and Microscopy. Science 1998, 280, 1732–1735
- Bachellier et al. 2020 Bachellier, N.; Verlhac, B.; Garnier, L.; Zaldívar, J.; Rubio-Verdú, C.; Abufager, P.; Ormaza, M.; Choi, D.-J.; Bocquet, M.-L.; Pascual, J. I.; Lorente, N.; Limot, L. Vibron-assisted spin excitation in a magnetically anisotropic molecule. Nature Communications 2020, 11, 1619
- Kondo 1964 Kondo, J. Resistance Minimum in Dilute Magnetic Alloys. Progress of Theoretical Physics 1964, 32, 37–49
- Scott and Natelson 2010 Scott, G. D.; Natelson, D. Kondo Resonances in Molecular Devices. ACS Nano 2010, 4, 3560–3579
- Hellerstedt et al. 2018 Hellerstedt, J.; Cahlík, A.; Švec, M.; de la Torre, B.; Moro-Lagares, M.; Chutora, T.; Papoušková, B.; Zoppellaro, G.; Mutombo, P.; Ruben, M.; Zbořil, R.; Jelinek, P. On-surface structural and electronic properties of spontaneously formed Tb2Pc3 single molecule magnets. Nanoscale 2018, 10, 15553–15563
- Mishra et al. 2020 Mishra, S.; Beyer, D.; Eimre, K.; Kezilebieke, S.; Berger, R.; Gröning, O.; Pignedoli, C. A.; Müllen, K.; Liljeroth, P.; Ruffieux, P.; Feng, X.; Fasel, R. Topological frustration induces unconventional magnetism in a nanographene. Nat. Nanotechnol. 2020, 15, 22–28
- Paaske et al. 2006 Paaske, J.; Rosch, A.; Wölfle, P.; Mason, N.; Marcus, C. M.; Nygård, J. Non-equilibrium singlet–triplet Kondo effect in carbon nanotubes. Nature Physics 2006, 2, 460–464
- Ternes 2015 Ternes, M. Spin excitations and correlations in scanning tunneling spectroscopy. New J. Phys. 2015, 17, 63016
- Jacob et al. 2021 Jacob, D.; Ortiz, R.; Fernández-Rossier, J. Renormalization of spin excitations and Kondo effect in open-shell nanographenes. Phys. Rev. B 2021, 104, 075404