Lower pressure phases and metastable states of superconducting photo-induced carbonaceous sulfur hydride
Abstract
Room-temperature superconductivity was recently discovered in carbonaceous sulfur hydride (C-S-H) close to 3 Mbar. We report significant differences in the superconducting response of C-S-H, with a maximum of 191(1) K, below a 1 Mbar. Variations in intensity of the C-H Raman modes reveal carbon content can vary between crystals synthesized with the same photo-induced method. Synchrotron single crystal x-ray diffraction identifies polymorphism with increasing degrees of covalency. These unique metastable states are highly sensitive to thermodynamic pathways.
In the search for superconductivity at ambient conditions, the current highest reported transition temperature, , is for a carbonaceous sulfur hydride (C-S-H) material with a of 288 K at 267 GPa.Snider et al. (2020) C-S-H belongs to a class of high-hydrogen content materials realized at over a megabar of pressure, whose investigation was ignited by the discovery of a 203 K at 155 GPa in hydrogen sulfide.Drozdov et al. (2015); Duan et al. (2014); Errea et al. (2016) These hydrogen-rich materials are of interest due to Ashcroft’s prediction that a hydrogen dominant alloy will act as a precompressed version of elemental hydrogen with lower transition pressures into the metallic and superconducting states.Ashcroft (2004) The precompressed hydrogen alloys are expected to share the same strong electron-phonon coupling, high Debye temperature, high hydrogen-related density of states at the Fermi level, and thus high- superconductivity as predicted for dense metallic hydrogen. The Wigner-Huntington phase of dense atomic metallic hydrogen has long been predicted to be a phonon-mediated type-II high- superconductor,Wigner and Huntington (1935); Ashcroft (1968) with dense molecular metallic hydrogen superconducting at even higher temperatures due to enhancements coming from electron-electron interactions.Richardson and Ashcroft (1997)
C-S-H, like hydrogen sulfide, is a covalent superhydride, wherein hydrogen is believed to form extended bonding networks with the other elements present.Pickard et al. (2020); Snider et al. (2021); Belli et al. (2021) This is opposed to the other main class of metal superhydides, which present as cage-like clathrate structures. The original theoretical attempts to identify candidate structures for C-S-H suggested a stoichiometry of CSH7. These structures consist of a molecular methane (CH4) unit encapsulated in an H3S perovskite-like sublattice with the rotational orientation of the CH4 unit relative to the H3S sublattice determining the symmetry.Cui et al. (2020); Sun et al. (2020) However, the most favorable structures were found to have insufficiently large values to justify their assignment as C-S-H. Other studies probed the possibility of carbon substitution for a sulfur in a hydrogen sulfide lattice,Ge et al. (2020); Hu et al. (2020) which obtained better agreement with experimental values. However, there are concerns over the validity of the structures owing to the inability of virtual crystal approximation (VCA) to capture the structural deformations arising from the doping.Wang et al. (2021a, b) Additional stoichiometries have been suggested, with the leading candidates being methane substituted hydrogen sulfide lattices with a C:S ratio closer to those of the VCA simulations than the 50% of the CSH7 structures.Wang et al. (2021b)
C-S-H was first synthesized via photochemistry from elemental precursors at 4 GPa. Raman spectroscopy revealed a rich phase diagram at low pressures (10s of GPa).Snider et al. (2020) This draws analogues to the H2S–H2 and CH4–H2 binary systems, which form extended van der Waals structures comprising the constituent molecules.Somayazulu et al. (1996); Strobel et al. (2011) In the absence of thermal annealing, as in the initial experiments, the compression pathway likely leads to a metastable state. Metastable states are known to be unreliably predicted using crystal structure prediction (CSP) methodologies. C-S-H has recently been experimentally synthesised using an alternative method, reacting elemental sulfur and methane-hydrogen fluid mixtures.Goncharov et al. (2021) In principal, this method permits greater control of methane concentration, although reported C-H Raman modes are comparably weak, and whether such routes lead to high superconducting states has yet to be studied.
To better understand the lower pressure phases of C-S-H, and motivated by the recent discovery of anomalous superconducting properties in YH6,Troyan et al. (2021) we investigate C-S-H in the sub-megabar regime to further understand the synthesis chemistry and the consequences of the thermodynamic pathways to metallization and superconductivity. We present electrical transport measurements in this previously unexplored pressure regime that reveal a remarkably high in some crystals, raising the question as to how these macroscopic quantum states emerge over such dramatically different pressure-temperature ranges. Using synchrotron single crystal X-ray diffraction (SC-XRD), the phase progression of C-S-H was determined to be , below a megabar, demonstrating an evolution from van der Waals to hydrogen bonding dominating ordering. We propose that the carbon content in C-S-H produced by photo-chemistry methods varies in each crystal synthesised. That variation directly affects the quantum properties for superconductivity, with subtle differences in packing densities.
Crystals of C-S-H are synthesized using the procedure of Snider et al. (2020) (full details in the Supplemental Materials).111See Supplemental Material at http://link.aps.org/supplemental/DOI for additional experimental and computational details. Ball-milled mixtures of elemental carbon and sulfur with dimensions about 15% of the diamond culet are placed into the sample chamber with a ruby sphere.Shen et al. (2020) The samples used in these runs are significantly larger, by roughly 3-10 times, using culets varying from 250 down to 100 m than in Snider et al. (2020). Gas phase H2 is loaded at 3 kbar.Smith et al. (2018) Samples are then pressurized to 3.7–4.0 GPa and excited for several hours using a 514 nm laser with power ranging from 10 and 150 mW depending on sample response. Raman spectroscopy confirms the transformation into C-S-H with the presence of C-H, S-H, and H-H Raman modes at 4 GPa.Snider et al. (2020) Larger crystals are less homogeneous than crystals formed using 30 m culets needed for ultra high pressures, as we observe variations of the appearance of C-H stretching modes to be spatially dependant using Raman spectroscopy. Samples are pressurized to 10 GPa after transformation and confirmation by Raman spectroscopy to avoid decomposition. Electrical transport measurements are carried out according to Snider et al. (2020). Synchrotron single crystal X-ray diffraction (SC-XRD) measurements were conducted at Sector 16, ID-B, HPCAT at the Advanced Photon Source ( = 0.34453 Å).
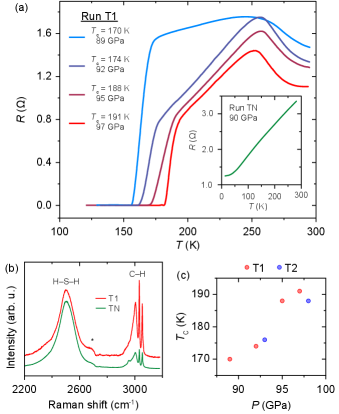
Figure 1 shows the results of the electrical transport measurements performed on three pristine C-S-H crystals at sub-megabar regimes. 138 GPa was the lowest pressure examined previously owing to a notion that the phase diagram and properties of C-S-H would be similar to hydrogen sulfide. In two separate runs, we observe a maximum of 191(1) K at 97(5) GPa (T1, plots in Figure 1a and red points in Figure 1c), and 188(1) K at 98(5) GPa (T2, blue points in Figure 1c), at roughly half the previously reported pressures for similar transition temperatures in Snider et al. (2020) (Fig LABEL:fig:SI-DTc in the SI). The onset of superconductivity begins at 170(1) K and 89(5) GPa (trend shown in Figure 1c). The shape of the superconducting dome shown in Figure 1c being distinct from the previous data suggests this dome comes from a different phase of C-S-H than the maximal =288 K. Also observed in Run T1 is the previously noted behavior of C-S-H to exhibit increasingly narrow / as a function of increasing pressure and , displaying the minimum / of 0.0373 at 97 GPa. (data can be found in Figure LABEL:fig:SI-DTc and Table LABEL:tab:SI2 in the Supplemental Materials). This superconducting transition is compared to the response of another C-S-H crystal, at a comparable pressure of 90 GPa, which, though metallized as evidenced by its decreasing resistance on cooling, does not becomes superconducting down to 10(1) K (Fig. 1 inset, run TN). The corresponding Raman spectrum of the superconducting C-S-H crystal exhibits stronger signal strength of the C-H stretching frequencies relative to the H-S-H mode (1.16 C-H/S-H). This ratio is comparably stronger than the same Raman features of Run TN when normalized, which exhibited a C-H/S-H ratio of only 0.27. The Raman spectrum shown in Fig 1b for run TN is more similar those reported previously.Snider et al. (2020); Goncharov et al. (2021)
All of the features for the different pressures measured for the superconducting C-S-H crystal from Run T1 feature a turning point around 250 K (Fig. 1a). A similar shape for was observed recently in dense hydrogen ( 320 GPa) when it was cooled from phase V to the (semi-)metallic phase III.Eremets et al. (2021) Above 250 K, C-S-H exhibits the temperature response of a finite gap system, whereas just below 250 K the temperature response is metallic. This behavior at 250 K likely results from either a structural or electronic phase transition. An electronic transition would likely not be be accompanied by a change in symmetry, and a structural transition in a hydride material might also be indistinguishable using XRD if the heavy atom sublattice does not re-order, as is the case for the to transition in H3S.Errea et al. (2016) Resistance continues to decrease with lowering temperature before a sharp drop to zero resistance as the critical temperature is crossed. Such a difference in to that of Snider et al. (2020) could be expected, as their thermodynamic approach to a superconducting state began from cooling the recently reported phase observed above 160 GPaGoncharov et al. (2021) rather than phase IV.
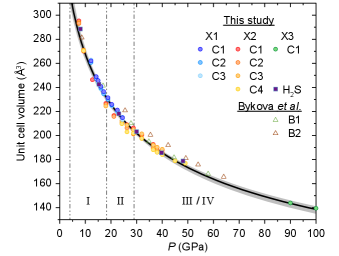
Figure 2 shows the volume-pressure response of 8 C-S-H crystals from three separate runs. Conical diamond with 80∘ apertures were used to enable a higher degree of completeness in SC-XRD. We observe subtle systematic differences in volume-pressure relations across the different crystals measured at the same thermodynamic conditions. The largest percent difference of volume was observed to be 2.9% at 28.9(5) GPa in Run X2 between crystals 1 and 4. The overall general volume trends for all of the C-S-H crystals measured are equal or lower than that of our own measurements on pure H2S+H2, which in turn is noticeably lower than that reported for C-S-H prepared from from mixtures of molecular H2S+CH4+H2 gases.Bykova et al.
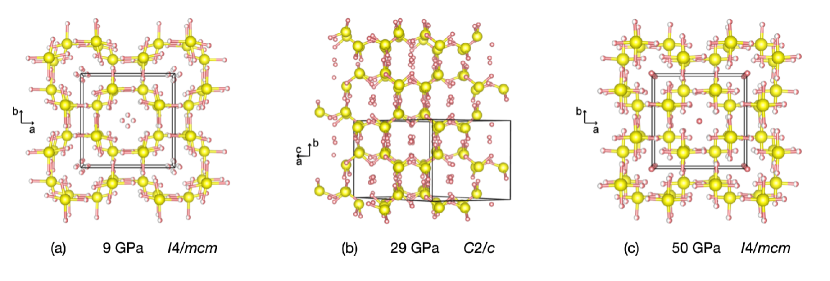
At low pressures (18 GPa), SC-XRD measurements confirm phase I Snider et al. (2020) as the Al2Cu-type structure () previously identified in molecular mixtures of CH4 and H2,Somayazulu et al. (1996) and in H2S and H2.Strobel et al. (2011); Bykova et al. The phase is inferred between 4 to 9 GPa as no observed change occurred in Raman spectroscopy. Due to insufficient C concentration or unique crystallographic placements, SC-XRD measurements are unable to resolve between C and S on the Wyckoff positions, thus Figure 3a displays only H2S units on the sites. Applying the Bernal-Fowler "ice rules"Bernal and Fowler (1933) to determine the H positions of these H2S units results in partially occupied Wyckoff positions in , and this constrains the H2S molecular units such that they are planar within {002} as in Strobel et al. (2011).
The 3.64 Å in-plane and 3.30 Å inter-plane nearest neighbor S–S distances are both within the hydrogen-bonded (H-bonded) dimerization distance (4.112 Å) of gas phase H2S molecules, implying hydrogen bonding contributes to the cohesion of the lattice.Das et al. (2018) A CSP study on the H–S system identified a modification which mostly varies from the H positions owing to the varied rotation of the molecular sub-units,Duan et al. (2014) which likely better reflect an instantaneous orientation in a thermalized sample as weak packing forces would enable the molecular sub-units to behave as weakly constrained rotors within their respective molecular volume. Comparing several planar arrangements of the hydrogen atoms as shown in Figure 3 versus the arrangement of the structure with density functional theory shows a 0.44 eV enthalpic preference for a non-planar hydrogen arrangement. This indicates C-S-H will have some non-planar arrangements of H2S molecular units to create weak H-bonding between the shorter 3.30 Å interplane nearest neighbor sulfur atoms.
Molecular dynamics (MD) equilibrations which kept the lattice and sulfur positions frozen at the values determined by XRD showed H2S units which were initially planar rotating such that one H atom pointed towards a S in another plane. Although the dynamics were performed on only a single unit cell, the H2S units are weakly constrained rotors going between hydrogen bonding configurations somewhat independently. This reinforces the favorability of interplane hydrogen bonding as well as implying rotational disorder to the phase at 300 K. The H2 molecules in this structure rotated and translated completely freely within the confines of their "Cu" position in the Al2Cu-type lattice, emphasizing their role as "guest" species within a guest-host structure.
At 18 GPa, C-S-H transforms into the phase shown in Figure 3b. This transition was observed for Runs X1 and the H2S loading, while there is variability in the appearance of this phase for the different crystals of run X2. A recent structural study also observed variation in the phase, and its occurrence was correlated to crystals with low CH4 concentrations. Goncharov et al. (2021) The phase resembles a monoclinically distorted version of the phase where the [101] direction of the structure roughly corresponds to the [001] direction of the structure. In both cases, that direction resembles a 2-dimensional pore formed by sulfur atoms interconnected by interplane hydrogen bonding that encapsulates the H2 molecules, and the views shown in Figure 3 are all oriented to look along this pore-like structure. The positioning of the hydrogens determined by XRD are reminiscent to what is observed in the low pressure dynamics snapshots.
Following the phase, C-S-H is observed to transform back into an structure at 29 GPa (shown in Figure 3c) which persists until our highest measurements at 100 GPa. Our measured phase transitions by SC-XRD agree well with those reported by Raman studies.Snider et al. (2020) However, XRD does not distinguish the phase III and IV previously reported around 45 GPa. This could be a reordering of hydrogen in the structure, that would be difficult to resolve in high-pressure XRD experiments. The noted electronic transition into a metallic phase evidenced by loss or Raman signal above 65 GPa does not display evidence of a structural transition from the solution up to our highest measurement at 100 GPa.
The hydrogen positions of the H2S units in Figure 3c are best modeled in a planar configuration. However, orientations with inter-plane hydrogen bonding were an order of magnitude more enthalpically favorable at 50 GPa than 9 GPa. The rotations of the H2S units observed with MD were highly concerted within the 50 GPa structure, and H2S units which were initially planar remained planar in MD simulations. These simulations show an increased importance of hydrogen bonding within the C-S-H lattice at higher pressures, indicating that molecular ordering is occurring between the low and high pressure structures and corroborating that further ordering is the likely difference between phases III and IV. It could be inferred from the constrained rotation that the monoclinic distortion seen in phase II likely arises from a reordering of the molecular orientations as the system densifies.
2nd-order Birch-Murnaghan equation of state (K0’ fixed to 4) are fit to each crystal and phase, and the resultant values for each may be found in the supplemental materials. K0 was found to range between 7.321 and 14.496 GPa for Runs X1 C3 and X2 C3. All data on phase III/IV was collected during Run X2. This, along with differences in the electronic response between crystals measured in this work (Figure 1), and especially between those and crystals reported previously in Snider et al. (2020) produced using a similar method, suggests a remarkable variability in C-S-H generated by photochemistry under pressure (Fig SX). Indeed, the effects of carbon doping on the superconducting properties of H3S have been investigated.Wang et al. (2021b)
A major challenge, that demands significant improvement, for the study of C-S-H is to ensure the control of the ratio of C:S but more even challenging is the product yield and controllable concentration of the constituent elements during the photo-induced reaction. The high-pressure photo-induced synthesis can also lead to nucleation of crystals that grow with preferred orientations resulting in difficulties manipulating crystals in a larger sample chamber. Consequently, ensuring that not only the chemically correct crystals grow, but the ideal part of the larger crystals make full contact with the transport leads is extremely challenging.
In conclusion, new transport measurements show a transition to a superconducting state with maximum of 191 K at pressures significantly lower than previously observed. SC-XRD marks a phase evolution of , , to finally at 18 and 29 GPa respectively. The absence of a measurable transition from phase III to IV from earlier Raman studies indicates that the transition is likely a reordering of the hydrogens, possibly molecular species in the pores, leaving the sulfur sublattice unchanged. Synthesis beginning from gas phase molecular precursors presents a promising path to greater control over the chemical homogeneity of C-S-H samples.Bykova et al. ; Goncharov et al. (2021) However, based on the path dependence metastability reported here, it needs to be confirmed that this route can produce superconducting C-S-H samples. Ambient conditions superconductivity will eventually be achieved with hydrogen dominant alloys produced with precise chemical control.
Acknowledgements.
This work supported by the U.S. Department of Energy, Office of Basic Energy Sciences under Award Number DE-SC0020303. AS and RD acknowledge Unearthly Materials for supporting this research. Portions of this work were performed at HPCAT (Sector 16), Advanced Photon Source (APS), Argonne National Laboratory. HPCAT operations are supported by DOE-NNSA’s Office of Experimental Sciences. The Advanced Photon Source is a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.References
- Snider et al. (2020) E. Snider, N. Dasenbrock-Gammon, R. McBride, M. Debessai, H. Vindana, K. Vencatasamy, K. Lawler, A. Salamat, and R. Dias, Nature 586, 373 (2020).
- Drozdov et al. (2015) A. P. Drozdov, M. I. Eremets, I. A. Troyan, V. Ksenofontov, and S. I. Shylin, Nature 525, 73 (2015).
- Duan et al. (2014) D. Duan, Y. Liu, F. Tian, D. Li, X. Huang, Z. Zhao, H. Yu, B. Liu, W. Tian, and T. Cui, Sci. Rep. 4, 6968 (2014).
- Errea et al. (2016) I. Errea, M. Calandra, C. J. Pickard, J. R. Nelson, R. J. Needs, Y. Li, H. Liu, Y. Zhang, Y. Ma, and F. Mauri, Nature 532, 81 (2016).
- Ashcroft (2004) N. W. Ashcroft, Phys. Rev. Lett. 92, 187002 (2004).
- Wigner and Huntington (1935) E. Wigner and H. B. Huntington, The Journal of Chemical Physics 3, 764 (1935).
- Ashcroft (1968) N. W. Ashcroft, Phys. Rev. Lett. 21, 1748 (1968).
- Richardson and Ashcroft (1997) C. F. Richardson and N. W. Ashcroft, Phys. Rev. Lett. 78, 118 (1997).
- Pickard et al. (2020) C. J. Pickard, I. Errea, and M. I. Eremets, Annual Review of Condensed Matter Physics 11, 57 (2020).
- Snider et al. (2021) E. Snider, N. Dasenbrock-Gammon, R. McBride, X. Wang, N. Meyers, K. V. Lawler, E. Zurek, A. Salamat, and R. P. Dias, Phys. Rev. Lett. 126, 117003 (2021).
- Belli et al. (2021) F. Belli, T. Novoa, J. Contreras-García, and I. Errea, Nature Communications 12, 5381 (2021).
- Cui et al. (2020) W. Cui, T. Bi, J. Shi, Y. Li, H. Liu, E. Zurek, and R. J. Hemley, Phys. Rev. B 101, 134504 (2020).
- Sun et al. (2020) Y. Sun, Y. Tian, B. Jiang, X. Li, H. Li, T. Iitaka, X. Zhong, and Y. Xie, Phys. Rev. B 101, 174102 (2020).
- Ge et al. (2020) Y. Ge, F. Zhang, R. P. Dias, R. J. Hemley, and Y. Yao, Materials Today Physics 15, 100330 (2020).
- Hu et al. (2020) S. X. Hu, R. Paul, V. V. Karasiev, and R. P. Dias, “Carbon-Doped Sulfur Hydrides as Room-Temperature Superconductors at 270 GPa,” (2020), arXiv:2012.10259 [cond-mat.supr-con] .
- Wang et al. (2021a) T. Wang, M. Hirayama, T. Nomoto, T. Koretsune, R. Arita, and J. A. Flores-Livas, Phys. Rev. B 104, 064510 (2021a).
- Wang et al. (2021b) X. Wang, T. Bi, K. P. Hilleke, A. Lamichhane, R. J. Hemley, and E. Zurek, “A Little Bit of Carbon Can do a Lot for Superconductivity in H3S,” (2021b), arXiv:2109.09898 [cond-mat.supr-con] .
- Somayazulu et al. (1996) M. S. Somayazulu, L. W. Finger, R. J. Hemley, and H. K. Mao, Science 271, 1400 (1996).
- Strobel et al. (2011) T. A. Strobel, P. Ganesh, M. Somayazulu, P. R. C. Kent, and R. J. Hemley, Phys. Rev. Lett. 107, 255503 (2011).
- Goncharov et al. (2021) A. F. Goncharov, E. Bykova, M. Bykov, X. Zhang, Y. Wang, S. Chariton, and V. B. Prakapenka, “Synthesis and structure of carbon doped H3S compounds at high pressure,” (2021), arXiv:2110.00038 [cond-mat.mtrl-sci] .
- Troyan et al. (2021) I. A. Troyan, D. V. Semenok, A. G. Kvashnin, A. V. Sadakov, O. A. Sobolevskiy, V. M. Pudalov, A. G. Ivanova, V. B. Prakapenka, E. Greenberg, A. G. Gavriliuk, I. S. Lyubutin, V. V. Struzhkin, A. Bergara, I. Errea, R. Bianco, M. Calandra, F. Mauri, L. Monacelli, R. Akashi, and A. R. Oganov, Advanced Materials 33, 2006832 (2021).
- Note (1) See Supplemental Material at http://link.aps.org/supplemental/DOI for additional experimental and computational details.
- Shen et al. (2020) G. Shen, Y. Wang, A. Dewaele, C. Wu, D. E. Fratanduono, J. Eggert, S. Klotz, K. F. Dziubek, P. Loubeyre, O. V. Fat’yanov, P. D. Asimow, T. Mashimo, R. M. M. Wentzcovitch, and other members of the IPPS task group, High Pressure Research 40, 299 (2020).
- Smith et al. (2018) D. Smith, D. P. Shelton, P. B. Ellison, and A. Salamat, Review of Scientific Instruments 89, 103902 (2018).
- Eremets et al. (2021) M. I. Eremets, P. P. Kong, and A. P. Drozdov, “Metallization of hydrogen,” (2021), arXiv:2109.11104 [cond-mat.mtrl-sci] .
- (26) E. Bykova, M. Bykov, S. Chariton, V. B. Prakapenka, K. Glazyrin, A. Aslandukov, A. Aslandukova, G. Criniti, A. Kurnosov, and A. F. Goncharov, Phys. Rev. B 10.1103/PhysRevB.103.L140105.
- Bernal and Fowler (1933) J. D. Bernal and R. H. Fowler, The Journal of Chemical Physics 1, 515 (1933).
- Das et al. (2018) A. Das, P. K. Mandal, F. J. Lovas, C. Medcraft, N. R. Walker, and E. Arunan, Angewandte Chemie International Edition 57, 15199 (2018).