Thermodynamic efficiency of atmospheric motion governed by Lorenz system
Abstract
The Lorenz system was derived on the basis of a model of convective atmospheric motions and may serve as a paradigmatic model for considering a complex climate system. In this study, we formulated the thermodynamic efficiency of convective atmospheric motions governed by the Lorenz system by treating it as a non-equilibrium thermodynamic system. Based on the fluid conservation equations under the Oberbeck–Boussinesq approximation, the work necessary to maintain atmospheric motion and heat fluxes at the boundaries were calculated. Using these calculations, the thermodynamic efficiency was formulated for stationary and chaotic dynamics. The numerical results show that, for both stationary and chaotic dynamics, the efficiency tends to increase as the atmospheric motion is driven out of thermodynamic equilibrium when the Rayleigh number increases. However, it is shown that the efficiency is upper bounded by the maximum efficiency, which is expressed in terms of the parameters characterizing the fluid and the convective system. The analysis of the entropy generation rate was also performed for elucidating the difference between the thermodynamic efficiency of conventional heat engines and the present atmospheric heat engine. It is also found that there exists an abrupt drop in efficiency at the critical Hopf bifurcation point, where the dynamics change from stationary to chaotic. These properties are similar to those found previously in Malkus–Lorenz waterwheel system.
I Introduction
Understanding climate systems is of increasing importance in coping with climate change; the utility of physics approaches in this regard has been demonstrated [1, 2, 3].
The climate system can be regarded as a nonequilibrium thermodynamic system, which exchanges the energy and entropy with the surroundings [3, 4, 1, 2, 5, 6]. Modeling the climate system as a heat engine driven by temperature differences has thus offered useful viewpoints [7, 2, 8]. Heat-engine-analogs were proposed by appropriately defining the heat inputs and outputs as well as the temperatures of hot and cold heat reservoirs in the climate system [9, 4, 10, 11]. The impacts of global warming on climate thermodynamics, including thermodynamic efficiency, were studied using an Earth-like climate model with the variation of the concentration [4], while the global entropy generation rate is often regarded as both a climate diagnostic and predictor [12].
Understanding atmospheric motion is an important element in comprehending complex climate systems. Convective atmospheric motions are caused by thermal imbalances owing to the difference in absorbed solar heat between upper and lower layers of the atmosphere. They can be interpreted as the result of mechanical work dissipated by viscosity and used as a way to decrease thermal imbalances by convective heat transport [2, 13, 14]. Historically, Saltzman developed a simplified model of time-dependent convective atmospheric motions and primarily solved it using a Fourier expansion [15]. Based on Saltzman’s model, Lorenz derived a set of nonlinear ordinary differential equations, known as Lorenz equations, which describe the motion of a specific mode of atmosphere, and discussed the stability of solutions [16]. The Lorenz equations are well known for yielding chaotic solutions under certain parameters and initial conditions, which may impact on the long-range weather forecasts [16]. Meanwhile, the thermodynamics of the Saltzman model and the Lorenz system are also studied via an excess work, which is related to the necessary work to displace the system from the stationary state [17]. The Lorenz model may provide a basic dynamical and thermodynamic framework for considering more complex models.
In addition to the atmospheric motion, Lorenz equations can be applied in different areas; examples include DC motors [18], chemical reaction systems [19], and a mechanical waterwheel model, known as the Malkus–Lorenz waterwheel [20, 21, 22]. Recently, the thermodynamic efficiency of the Malkus–Lorenz waterwheel system was numerically and theoretically studied, and its maximum efficiency has been derived [22]. It has also been found that the efficiency shows an abrupt drop at the point where the dynamics change from stationary to chaotic, and this can be applied to the more generic systems described by Lorenz equations other than the Malkus–Lorenz waterwheel system.
In this study, stimulated by [22], the thermodynamic efficiency of atmospheric motion is studied based on Saltzman’s model and Lorenz equations. Taking the mathematical similarities into consideration, it can be reasonably assumed that this efficiency is similar to the one in [22]. An analysis of the entropy generation rate will also bring out the difference between the efficiencies of the atmospheric heat engines and those of conventional heat engines.
The remainder of this paper is structured as follows: A review of the setup of the Lorenz system is given in Sec. II. Subsequently, the thermodynamic efficiency and the entropy generation rate of atmospheric motion is calculated in Sec. III. To obtain efficiency, the heat flux and work necessary to maintain the atmospheric motion are determined. Then, in Sec. IV, a numerical calculation of efficiency and entropy generation rate is applied for one set of parameters, which can lead to chaotic dynamics with a relatively high Rayleigh number. Finally, we summarize the present study and discuss it in Sec. V.
II Lorenz system
First, we review the setup of the Lorenz system [15, 16]. It was originally derived from the Oberbeck–Boussinesq approximation of fluid conservation equations, where the density variations are neglected, except when they give rise to a gravitational force [7]. Let us consider the atmospheric motion between two parallel horizontal plates with distance , where each plate is externally heated to maintain its temperature such that the temperature difference between the plates is maintained constant. Assuming that the atmospheric motion is restricted to a two-dimensional - plane with vanishing velocity in -direction, the Navier–Stokes equations and the thermal convection equation in - plane under the Oberbeck–Boussinesq approximation can be given as follows [15]:
| (1) | ||||
| (2) |
Here, and are the velocities in - and -directions, respectively, satisfying the incompressibility condition in - plane. and are the temperature and pressure divided by density, respectively. The constants , , , and denote the gravitational acceleration, coefficient of thermal expansion, kinematic viscosity, and coefficient of thermal diffusivity, respectively.
Owing to the incompressibility condition, the stream function can be introduced for two-dimensional motion, where the velocities in - and -directions are expressed as and , respectively. We also introduce , which is the nonlinear part of temperature :
| (3) |
It also denotes the departure of temperature from that in a state of vanishing convection [16], where . Then, the governing equations (1) and (2) can be rewritten in terms of and as [15]
| (4) |
| (5) |
where
| (6) |
is the Jacobian operator
Using the method developed in [23] by scaling the length in , time in , stream function in , and temperature in , a dimensionless version of Eqs. (4) and (5) can be given as
| (7) |
and
| (8) |
with Prandtl number and Rayleigh number . Here, superscript “ ∗ ” denotes the dimensionless replacement of the corresponding variable.
Naturally, for both the upper and lower boundaries, we impose
| (9) |
because the temperatures of the two parallel horizontal plates are maintained constant. Moreover, we impose the following free boundary conditions on for tractability [16]:
| (10) | ||||
By considering the boundary conditions Eqs. (9) and (10), modes and are available for and , where is the dimensionless wavelength in -direction, and and are the wave numbers in - and -directions, respectively. These modes imply the repetitive convective cells with each height and length .
Lorenz discovered that for a specific set of modes,
| (11) | ||||
where
| (12) |
denotes the critical Rayleigh number, , and are governed by the following equations.
| (13) | ||||
where , , and [16]. Equation (13) is known as the Lorenz equation. There is one stable fixed point,
| (14) |
when and other two fixed points
| (15) |
when [16, 20]. For , fixed point (14) is unstable, whereas fixed points (15) are stable if [16, 20]. However, if , fixed points (15) are only stable when and become unstable when . The strange attractor called “Lorenz attractor” appears [20]. A supercritical pitchfork bifurcation occurs at , and if , a subcritical Hopf bifurcation occurs at [20]. It can be proven that for any available when .
A mechanical waterwheel model is (partly) governed by the Lorenz equations known as the Malkus–Lorenz waterwheel, in which water flows into a sloping wheel in a symmetry mode and leaks at a constant rate, causing the wheel to rotate against friction [20, 21, 22]. This waterwheel model can be regarded as a generalized heat engine because it absorbs potential energy from the inflow and works against friction to maintain its rotation [22]. The thermodynamic efficiency is given by
| (16) |
when the system approaches stable fixed points (15) and is bounded by the maximum efficiency irrespective of whether the dynamic is chaotic [22]. is the redefined Rayleigh number in the waterwheel model.
The thermodynamic efficiency of the Malkus–Lorenz waterwheel is related to the Rayleigh number, which, in the atmospheric motion situation, is related to the temperature difference . As in the case of the Malkus–Lorenz waterwheel, it is also necessary for the atmosphere to absorb energy, particularly heat energy, to maintain its motion. Thus, it is reasonable to regard atmospheric motion as a heat engine and the thermodynamic efficiency, which may also be bounded by maximum efficiency, can be expressed by the Rayleigh number . In other words, the efficiency may be similar to that expressed in Eq. (16) when the dynamics of the system are stationary.
Moreover, the thermodynamic efficiency of free convection is qualitatively given as:
| (17) |
where is the length scale of the convection system (for the situation considered in this study, ) and is the constant-pressure specific heat [7]. This expression may be related to maximum efficiency .
III Thermodynamic efficiency
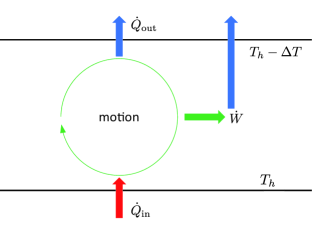
Figure 1 shows a schematic of atmospheric motion. The heat is absorbed per unit time at the hot boundary , and a part of it is converted to work per unit time to maintain the motion. The heat is dissipated per unit time at the cold boundary . The efficiency can be defined as:
| (18) |
It must be noted that is usually dissipated again, such that [7].
Moreover, it is necessary to revert to the governing equations (1) and (2) before calculating heat absorbed per unit time , which is related to heat flux , and work per unit time .
III.1 Heat flux
It is easy to obtain the heat flux that satisfies
| (19) |
from Eq. (2) with the incompressibility condition in - plane. Here, can be chosen as
| (20) |
which is the total heat flux including convection term where and conduction term [15]. In particular, in -direction, we have
| (21) |
at the boundary is related to the absorbed and dissipated heat, respectively. By applying the mode in Eq. (11) and integrating at both the hot boundary and the cold boundary , the absorbed heat per unit time can be calculated as follows:
| (22) |
whereas the dissipated heat per unit time is
| (23) |
Here, , which implies that the work per unit time to maintain the motion is finally dissipated.
III.2 Work to maintain the atmospheric motion
The kinetic energy per unit mass is defined as follows:
| (24) |
Combined with Eq. (1), the energy conservation equation can be expressed as follows:
| (25) |
Here, is related to the necessary work per unit time to maintain the motion, is the convection term with no energy input and output, as when considering the boundary conditions (9) and (10), and can be expressed by an “available potential energy” when considering the average over the entire fluid [15]. It should be noted that the mechanical work is assumed to be only dissipated as the frictional heat. Although other factors, such as phase changes in the water cycle, also play important roles in a climate system [24, 25, 26], they do not appear in the governing equations (1) and (2).
Considering the mode in Eq. (11), the necessary work per unit time to maintain the motion can be calculated by integrating over the entire motion space as
| (26) |
III.3 Efficiency
It appears that by using in Eq. (22) and in Eq. (26), the thermodynamic efficiency can be calculated directly using Eq. (18). However, these dynamics may become chaotic under certain parameter sets. Thus, it is necessary to determine whether the dynamics are stationary or chaotic to obtain efficiency.
The case is straightforward if the dynamics approach a fixed point. Using Eqs. (22) and (26), the efficiency can be expressed as
| (27) |
The fixed point (14) is approached if , whereas the fixed points (15) is approached if and , or if and . Thus, the efficiency can be calculated as:
| (28) |
For chaotic dynamics, if and , it cannot be derived as a closed expression for efficiency because the dynamics evolve through the chaotic attractor [22]. Nevertheless, and can be averaged because the limit set is densely covered by a particular trajectory [22]. The average of a function is defined as follows:
| (29) |
Thus, the efficiency can be expressed as
| (30) | ||||
III.4 Entropy generation rate
The present system can be considered to be in a steady state with in a statistical sense [27], no matter whether the dynamics is stationary or chaotic. Therefore, the entropy generation rate of the total system should be equal to the entropy increase rate in the surrounding environment [27]:
| (31) |
By time averaging Eq. (31), we have
| (32) | ||||
for both stationary and chaotic dynamics, where we have defined the parameter with a dimension of the entropy generation rate as
| (33) |
Here, in addition to , we used Eq. (22) and the definition of Rayleigh number , and assumed
| (34) |
due to the Oberbeck–Boussinesq approximation, which claims . Especially, when the dynamics is stationary, Eq. (32) becomes
| (35) |
when approaching to the stable fixed point in Eq. (14), or
| (36) |
when approaching to the stable fixed point in Eq. (15).
IV Numerical calculation
IV.1 Efficiency
We easily find that the efficiency in Eq. (28) for the stationary dynamics is bounded from above by , for example, for . Although the expression of the efficiency cannot be derived as a closed one under chaotic dynamics, we may expect that the efficiency shares the same upper bound, , as in the case of stationary dynamics, which will be shown in Appendix A.
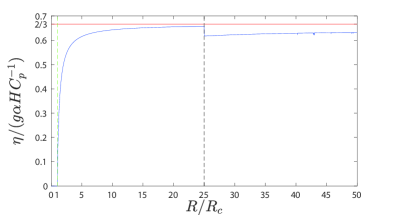
Figure 2 shows the numerical calculation results of the non-dimensionalized efficiency , plotted as a blue solid line. The necessary parameters were chosen as and , making it possible for the dynamics to become chaotic. The two dashed lines in the figure represent the pitchfork bifurcation point at (green dashed line) and subcritical Hopf bifurcation point (black dashed line). The stable fixed point (14) is approached when and the efficiency vanishes, whereas a stable fixed point (15) is approached when . The efficiency is described by Eq. (28) in stationary dynamics and is calculated using Eq. (30), when in chaotic dynamics.
Except for the discontinuous point at , the efficiency increases as increases when . Because , the efficiency increases as the temperature difference increases. This type of behavior is commonly observed in the efficiency of heat engines operating between two heat reservoirs at different temperatures, such as the Carnot heat engine [22]. It is crucial to recognize the increasing complexity due to various additional factors in considering the large-scale climate regime. For example, moisture plays an important role in the efficiency of the climate system [24, 25, 26], which is not captured by the model presented in this study.
Moreover, regardless of whether stationary or chaotic, is bounded by (red solid line), implying that (see Appendix A for the derivation)
| (37) |
Although the value of the upper bound is not explicitly mentioned, there should be no concern regarding the forbidden situation because is limited. Otherwise, the Oberbeck-Boussinesq approximation does not hold because density differences cannot be ignored [7]. In fact, should be very small according to the qualitative description in [7].
IV.2 Entropy generation rate
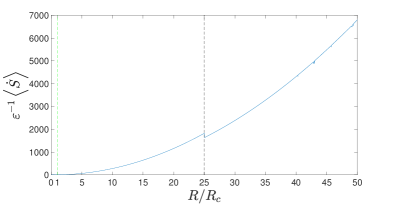
Figure 3 shows the numerical results of the entropy generation rate . The parameters in the Lorenz equations (13) are chosen as and .
Basically, no matter whether the dynamics are stable or chaotic, in Eq. (32) monotonically increases as increases; especially, in Eq. (36) for the stationary dynamics behaves as
| (38) |
The exception is the Hopf bifurcation point , where the abrupt drop of is evident.
Upon comparing Figs. 2 and 3, it is evident that, for both stationary and chaotic dynamics, the efficiency increases despite the increase of the entropy generation rate. This is consistent with the results of [22], but is clearly different from the conventional thermodynamic efficiency of heat engines, where the increase of irreversibility generally reduces the efficiency. This is attributed to the fact that the generated work in the atmospheric heat engine is eventually dissipated as heat into the cold heat reservoir.
V Summary and Discussion
In this study, the thermodynamic efficiency of the atmospheric motion governed by the Lorenz system was determined and calculated. It is shown in Sec. III that the heat input from the hot boundary is equal to the heat output to the cold boundary because the work, which is converted from a part of the heat input, dissipates again [7].
An upper bound of the efficiency exists when the Rayleigh number varies, which is similar to the results in [22], regardless of whether the dynamics are chaotic or not. The upper bound has the same structure as that given qualitatively in [7].
For the parameter sets that can cause the dynamics to be chaotic, an abrupt drop in efficiency occurs at Hopf bifurcation. This drop, which is because of the discontinuity of , is also discovered in the Malkus–Lorenz waterwheel system [22].
As for the entropy generation rate, the similar drop as the efficiency also happens at the Hopf bifurcation point.
It is in our expect to find these similarities when compared with the Malkus-Lorenz waterwheel system because they are commonly described by the Lorenz equations. However, the efficiency and the entropy generation rate in this study are not exactly the same as those in [22]. Both the “Rayleigh number” and “Prandtl number” are variable in the Malkus–Lorenz waterwheel system by adjusting friction rate “” and water inflow mode, but the Prandtl number is fixed here for the specific fluid, and Rayleigh number can be changed by only adjusting . Moreover, the length scale is limited because of the Oberbeck–Boussinesq approximation [7]. Despite these differences, such similarities are interesting.
Notably, as a simplified model, the present model governed by the Lorenz system has certain limitations. Its application in the large-scale climate modeling is challenging because of the Oberbeck–Boussinesq approximation. Moreover, the frictional dissipation in the Saltzman model accounts only for a small fraction of the entropy generation rate, where the latent heat transport given by moisture and its phase changes are not taken into consideration [24, 25, 26]. Further, in addition to the vertical convection considered in the Saltzman model, horizontal heat transportation, which is dominant at the midlatitude, also plays an important role [28, 29].
Despite these limitations, the present study makes significant contributions to the understanding of the complex climate system from a thermodynamics perspective. Further, we intend to add other factors to this simple model and find their properties in both dynamics and thermodynamics. We expect that similar behaviors, such as the discontinuities of efficiency and entropy generation rate at certain bifurcation points, still exist.
Acknowledgements.
This work was supported by JSPS KAKENHI Grant Numbers 19K03651 and 22K03450.Appendix A Decrease of and derivation of Eq. (37)
The average is a linear operator, and for a limited function , it can be proven that
| (39) |
| (40) |
and
| (41) |
with equality if and only if is a constant.
Note that the variables in the Lorenz equations in Eq. (13) are limited because they approach a stable fixed point or evolve through a chaotic attractor [22]. Applying Eq. (39) to the third equation in Eq. (13) gives
| (42) |
Multiplying on both sides of the first equation in Eq. (13), and applying Eq. (39), we obtain
| (43) |
From Eqs. (42) and (43), we have
| (44) |
Therefore, from Eq. (44), the efficiency Eq. (30) can be re-expressed as:
| (45) |
The decrease in at indicates a decrease in at the same point.
Multiplying on both sides of the second equation and on both sides of the third equation of Eq. (13), followed by applying Eq. (39), gives the following
| (46) | ||||
and
| (47) |
Equation (41) shows with equality if and only if the dynamics are stationary. Thus,
| (48) |
Consider the following relationship:
| (49) | ||||
with equality if and only if the dynamics are stationary, which implies that
| (50) |
With Eq. (48) and Eq. (50), we obtain:
| (51) |
For ,
| (52) |
It is worth noting that is the value of at fixed points (15), when the dynamics are stationary, and when the dynamics are chaotic. This will lead to the drop in both efficiency and entropy generation rate, which is consistent with the numerical calculations (see Fig. 2 and 3). Moreover, the abrupt drop in the numerical calculations implies the discontinuity of at , which is the subcritical Hopf bifurcation point. Finally, by applying for chaotic dynamics to Eq. (45), we have
| (53) |
Thus, the efficiency for the chaotic dynamics has been proved to be lower than the efficiency for the stationary dynamics in Eq. (28) corresponding to Eq. (15), and we have thus derived Eq. (37).
References
- Ghil and Lucarini [2020] M. Ghil and V. Lucarini, Rev. Mod. Phys. 92, 035002 (2020).
- Singh and O’Neill [2022] M. S. Singh and M. E. O’Neill, Rev. Mod. Phys. 94, 015001 (2022).
- Lucarini et al. [2014] V. Lucarini, R. Blender, C. Herbert, F. Ragone, S. Pascale, and J. Wouters, Reviews of Geophysics 52, 809 (2014).
- Lucarini et al. [2010] V. Lucarini, K. Fraedrich, and F. Lunkeit, Atmos. Chem. Phys. 10, 9729 (2010).
- Lucarini [2009] V. Lucarini, Phys. Rev. E 80, 021118 (2009).
- Peixoto et al. [1991a] J. P. Peixoto, A. H. Oort, M. De Almeida, and A. Tomé, Journal of Geophysical Research: Atmospheres 96, 10981 (1991a).
- Tritton [2012] D. J. Tritton, Physical fluid dynamics (Springer Science & Business Media, 2012).
- Lorenz [1955] E. N. Lorenz, Tellus 7, 157 (1955).
- Johnson [2000] D. R. Johnson, in International Geophysics, Vol. 70 (Elsevier, 2000) pp. 659–720.
- Bannon [2015] P. R. Bannon, Journal of the Atmospheric Sciences 72, 3268 (2015).
- Bannon and Lee [2017] P. R. Bannon and S. Lee, Journal of the Atmospheric Sciences 74, 1721 (2017).
- Gibbins and Haigh [2020] G. Gibbins and J. D. Haigh, J. Atmos. Sci 77, 3551 (2020).
- Lorenz [1967] E. N. Lorenz, World meteorological organization 161 (1967).
- Peixoto et al. [1991b] J. P. Peixoto, A. H. Oort, M. De Almeida, and A. Tomé, Journal of Geophysical Research: Atmospheres 96, 10981 (1991b).
- Saltzman [1962] B. Saltzman, J. Atmos. Sci 19, 329 (1962).
- Lorenz [1963] E. N. Lorenz, J. Atmos. Sci 20, 130 (1963).
- Velarde et al. [1994] M. G. Velarde, X.-l. Chu, and J. Ross, Physics of Fluids 6, 550 (1994).
- Hemati [1994] N. Hemati, Trans. Circuits Syst. I 41, 40 (1994).
- Poland [1993] D. Poland, Physica D 65, 86 (1993).
- Strogatz [2018] S. H. Strogatz, Nonlinear dynamics and chaos: with applications to physics, biology, chemistry, and engineering, 2nd ed. (CRC press, 2018).
- Malkus [1972] W. V. R. Malkus, Mem. Roy. Sci. Liège 4, 125 (1972).
- López et al. [2023] Á. G. López, F. Benito, J. Sabuco, and A. Delgado-Bonal, Chaos, Solitons & Fractals 172, 113521 (2023).
- Malkus and Veronis [1958] W. V. R. Malkus and G. Veronis, Journal of Fluid Mechanics 4, 225 (1958).
- Lembo et al. [2019] V. Lembo, F. Lunkeit, and V. Lucarini, Geoscientific Model Development 12, 3805 (2019).
- Pauluis and Held [2002a] O. Pauluis and I. M. Held, J. Atmos. Sci 59, 125 (2002a).
- Pauluis and Held [2002b] O. Pauluis and I. M. Held, J. Atmos. Sci 59, 140 (2002b).
- Ozawa et al. [2003] H. Ozawa, A. Ohmura, R. D. Lorenz, and T. Pujol, Reviews of Geophysics 41, 1018 (2003).
- Lucarini et al. [2011] V. Lucarini, K. Fraedrich, and F. Ragone, J. Atmos. Sci 68, 2438 (2011).
- Juckes [2000] M. Juckes, Quarterly Journal of the Royal Meteorological Society 126, 1065 (2000).